Make it Simple, Make it Right
Clinicians and surgeons respond to simple innovations that make a device easy to use. But achieving such simplicity isn’t always easy.
April 1, 2007
MDEA 2007
Seymour Cray, inventor of the Cray supercomputer, once remarked, “I like simple. If it's complex, then I don't understand it.”
For medical devices, designers strive to simplify products for simple reasons. “Healthcare is intrinsically very complex,” says Medical Design Excellence Awards (MDEA) juror Ogan Gurel. Gurel is chair of Aesis Research Group. “Devices in the care process must absolutely be as simple as possible.” In hospitals and other healthcare settings, a simple device may prove more popular than an unnecessarily complex one. “Devices that are simple and intuitive at the first use will be reused because of a positive first-time experience,” says juror Michael Schollmeyer, director of clinical research at CHF Solutions.
Beyond the need for devices to be easy to use, users also relate to the psychological aspects of simplicity. Juror Michael Wiklund explains it this way: “I closely associate simplicity with quality and innovation. The best products become visually and interactively simpler over time.” Wiklund is president of Wiklund Research & Design.
But simplicity is often elusive. And more often than not, a final device that is simple requires significant planning and effort, neither of which is easy to accomplish.
“Converging on simple solutions is actually quite hard and might lead to considerable internal complexities that the user will never see,” says Wiklund. “It takes commitment and resources to conduct the necessary design and engineering explorations to discover simple solutions and bring them into being.”
If successful, a company can create a product that is elegant, inexpensive, and intuitive. After that, it might be worthy of a Medical Design Excellence Award, as are the following products.
Priced to Move
Medical devices are justifiably expensive. They require significant research, engineering, and regulatory approvals, and they are made from costly materials. Most device companies rely on reimbursement to make their devices affordable to those who need them. But if a device does not qualify, or is considered elective, obtaining reimbursement can be difficult. In such cases, companies must do what they can to lower the cost of the device while maintaining the quality expected by the clinical community. These constraints can often result in a graceful and inexpensive device.
|
The Xtensor impressed several judges with its simple-but-effective design and its low price point. |
The Xtensor, by Clinically Fit Inc. (Melville, NY), is a therapeutic conditioning device to correct upper-extremity disorders. It applies resistance when a user opens his or her hands and allows a natural range of motion while stimulating the extensor muscles, tendons, and finger joints.
The Xtensor weighs only 5 oz and can be used on either hand. It is meant to provide rehabilitation from corrective surgery or trauma, but it can also be used to prevent repetitive-stress injuries or tendonitis. “We estimate that one in five people domestically have hobbies or jobs that create a need for exercising the extensor muscle,” says Scott Kupferman, CEO of Clinically Fit.
The device comprises a wrist wrap and palm support, with adjustable finger bands. The bands are made from a custom-blended thermoplastic elastomer with up to 700% elasticity. These bands are conically shaped and are formed into three sizes. To maintain resistance, the conical shape prevents the bands from slipping down the fingers, and the three sizes are designed to accommodate almost any hand, regardless of gender, weight, age, or vocation.
“Not everyone's hands move the same way,” Kupferman says. In addition, he says, different people have different capabilities. “Treadmills, for example, can be used by a healthy person or a person who has just had a heart attack and needs to get stronger,” he says. “We needed to be able to accommodate a universal range of motion and various levels of fitness.”
MDEA jurors were impressed with the Xtensor. “It does not look like a medical device. It looks like sports equipment,” says Walter Greenleaf, president of Greenleaf Medical. The designers deliberately tried to achieve a sports-minded look to aid in compliance of use. “Also, it's only $30 retail, so it's a good package for the price,” Greenleaf says. Several jurors also commented on how the design team used cost-saving materials and used low-but-effective technology, such as the tendon-like rubbery cords that can be locked into three different positions to control tension.
“The Xtensor was one of my favorite products. I was impressed with its level of design refinement,” says Wiklund. “This kind of product usually looks like a torture device made in a machine shop. I suspect that patients would develop a positive first impression of the device, which might increase their compliance with associated exercising regimens.”
The device meets a significant need for extension elongation, and the company stressed that it is the only type of product for arm extensor muscles at this price point. The Xtensor can be used either in a clinician's office or at home.
Another inexpensive product that also met high design requirements was the Thermoplastic Drill Template Set for single and free-end tooth replacement, manufactured by
Institut Straumann AG (Basel, Switzerland). “This product is representative of the trend to use guided surgical techniques to complete a procedure correctly without revision,” says juror Mary Beth Privitera. Privitera is a codeveloper and faculty member of the medical device innovation and entrepreneurship program at the University of Cincinnati.
The set enables a dental surgeon to quickly fabricate an accurate radiographic and surgical guide for proper implant placement. It marks the site of the implant and increases precision for cutting during implant bed preparation.
The device consists of a polymer template with a titanium drill sleeve. The polymer softens in hot water so that it can be molded over a patient cast, enabling the drill sleeve to be accurately positioned. Once the polymer hardens again, the device is cold sterilized and then used as a guide during surgery.
|
The Thermoplastic Drill Template Set from Institut |
“The Thermoplastic Drill Template Set illustrates how a simple device can be designed to provide the positioning precision required for successful and minimally traumatic dental implant preparation,” says juror Craig Jackson. Jackson is president of Hemosaga Diagnostics. The device has minimal parts and is inexpensively manufactured using CNC milling and injection molding. But its simplicity belies the device's important function in the surgical procedure.
Osteotomy must be precise to ensure proper fit and cosmetic result for a prosthetic device. Improperly drilled holes can cause injury to a nerve bundle or to the maxillary sinus. Competitive methods for identifying the drill hole include using a vacuum-formed resin sheet that must be peeled away from the cast, or farming the task out to an outside laboratory. Both practices are time consuming. “I really wanted to make a product that didn't take too long and could be done just before the surgery,” explains Brian Tang, president of Applied Dental, who designed the template. “We were determined to get a good price point, but the time factor was even more important.” Tang also points out that the template shows up on a CT scan so that users can further verify the angle before surgery.
The drill set is unique because any dental surgeon can use it. The price is $150 for a set. Privitera found the price point and the method important. “They really took an inexpensive and elegant approach to this problem,” she says. “I don't think the procedure is reimbursed, so anything you can do to cut costs in such a simple way is a benefit to patients.” Tang adds that industry is realizing the value of these implants and that insurers are slowly beginning to grant coverage.
Conveniently Located
|
The Ospol dental implant system includes a well-designed and clearly organized package system that promotes sterility and |
When a product is valuable, the packaging conveys much of that worth. Such is the case with the Ospol dental implant system by Ospol AB (Malmö, Sweden), which incorporates an organized package system for dental implants. “What is in the package is, of course, critical especially for a medical device product, but most product development in the industry seems to have stopped there,” says Ospol's CEO Hans Berglund. It is not enough, he says, to consider only whether a product performs well, clinically speaking.
What makes the system award worthy is its attention to helping surgeons keep track of these small devices, because each part has its own location. According to the company, Ospol packaging is used to hold dental implants and tools at dental clinics to provide safe and easy management in a sterile environment. The system consists of a titanium tray that holds all the parts and tools. Within this tray are the parts (screw-shaped titanium dental implants), which are also encased in individual sterile pods with pull-off lids. Within that pod is a secondary inner case made from titanium to protect the implant from plastics.
The product is designed to fit with all parts and to support all phases of the implant system to which it belongs. The designers took care to select materials and processes that support sterility and that do not interfere with ease of use. For example, the lids for the inner cases are made from Tyvek so that they will not tear. In addition, the lids are blister welded to reduce waste. The designers opted not to use any color other than white for all parts of the packaging, to prevent colors from transferring harmful substances to the implant. The inner titanium package can be removed from the plastic for recycling, and the entire package can be E-beam sterilized. Berglund explains that handling of the product was one of the areas that showed clear inefficiencies with existing brands. The Ospol, he says, combines functional values with design values.
Greenleaf was particularly impressed with the way everything fit together. “The system is so organized that you can't mess it up,” he says. “I like that the pods are easy to open, and they can even be picked up by an instrument.”
Juror Herbert Voigt points out that the organization is a significant benefit for oral surgeons, but it also relates to patient benefits. “A patient feels more comfortable seeing a dentist in control of all implant devices,” he says. In addition, Voigt, who is president of the American Institute for Medical and Biological Engineering, points out that each pod is bar coded to ensure patient safety.
“It's an elegant solution. The designers have thought out the product access and incorporated good ergonomics,” says juror Pascal Malassigné, a professor of industrial design at the Milwaukee Institute of Art and Design.
|
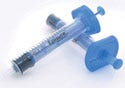
Indigo Orb's Episure Epidural Syringe can easily replace currently used epidural syringes because it is similar in size, shape, and method of use. |
Often, the key to knowing you have an award-winning device in your hand is when you ask, “Why didn't I think of that?” During the judging, Schollmeyer asked that question about the Episure AutoDetect epidural syringe from Indigo Orb Inc. (Irvine, CA). “It's so simple,” he says.
The Episure is an epidural syringe that incorporates a spring-loaded loss-of-resistance function that helps users verify when the needle has reached the appropriate epidural space. When the correct location is reached, the plunger automatically releases.
“We wanted to standardize the practice of administering an epidural,” says Bobby Shah, vice president of sales and marketing for Indigo Orb. “Right now, there are no two clinicians who do it the same way.”
The skill is difficult to teach, he says. The Episure enables the administrator to use both hands for placement, rather than having to have a thumb on the back of the syringe to feel for loss of resistance. Such maneuverability could help precision and patient care. “We think it is a great teaching tool,” Shah says. “It could be beneficial for new resident doctors.”
According to the company, improving the precision of the device could help reduce inadvertent spinal taps or dural punctures. This type of error can cost thousands of dollars to correct. “I really like that it takes what was once a tactile feedback system and turns it into a visual cue,” says juror Edmond Israelski. “No longer does the clinician need to risk patient safety by solely relying on the ability to feel the loss of resistance during epidural needle insertion.” Israelski is human factors program manager for Abbott Laboratories.
The designers made the syringe easy to adopt because it is similar to currently used devices. Therefore, clinicians do not have to change their epidural process. “It is such a simple concept, and users don't have to compromise their own skills,” Shah explains.
In addition, the product is manufactured at the same price as similar syringes, but can be sold at a premium because it may have enhanced clinical benefits. “The design of the Episure Epidural Syringe assembly illustrates that recognizing human factors design considerations can lead to superior products,” says Jackson.
Jurors were also intrigued by the potential for faster and safer epidural administration. “Giving an epidural is a risky procedure that could negatively affect approximately one out of 100 patients,” explains juror Robert Virag. “So if this can help, it could be a valuable tool. However, it still needs clinical studies and more work to prove its benefits.” Virag is founder and principal of Trifid Medical Group and Alveoli Medical.
Easy to Operate
A hallmark of a simple device is that it reduces the parts needed to make it work. Furthermore, the device should also be one that can be used instinctively; the user may not even have to read the directions.
|
By providing a large and easy-to-understand display, Hill-Rom's Vest airway clearance system encourages user comfort. |
One award winner that embodied this intuitive use was the Vest airway clearance system, Model 205, from Hill-Rom Company, Inc. (Charleston, SC). It uses a high-frequency chest-wall oscillation method for clearing retained secretions caused by acute and chronic respiratory conditions.
A vest, which is placed around the patient, is attached to two hoses. These hoses are connected to a console on a rolling stand. The console can be programmed for duration, intensity, and cycles. It is meant to replace or augment manual chest physiotherapy (a practice that involves having the caregiver apply repetitive pressure through cupped hands). Such therapy can often last from 30 to 60 minutes and is physically demanding for caregivers. The Vest system frees time and energy for caregivers so that they can see to the patient's other needs.
One way to help simplify a product is to adapt and update existing technology. More than 80% of the technology Hill-Rom used for the Vest already existed. This means the company was able to minimize capital tooling and manufacturing costs. According to Chad Boerst, director of operations and development, the approach helped the company get to the market more quickly. “The path we took saved a year for the overall project,” he says. Boerst explains that in addition to saving manufacturing costs, the company was able to keep the same vendors and was able to maintain testing parameters for the device.
Best of all, with the technology and components under control, designers were able to put their energy into making the device easy to use and approachable. “So many medical devices intimidate first-time users. However, the Vest airway clearance system is benign looking,” Wiklund explains.
The company achieved this look by modifying colors to blue and beige and by using a large screen with only a few large-font buttons to push. “Even though the stand was built after the device, the designers took pains to make sure that the colors went together. That is a nice design aesthetic,” Wiklund says. Boerst explains that the colors were part of the company's branding campaign.
The designers of the Vest system also considered patient needs. “We wanted to provide customers with usability and versatility,” says Boerst. This device is meant for patients in acute and long-term-care environments, and it is designed to ease the patient into the therapy. For example, instead of inflating immediately upon pressing the on button, the vest fills slowly with air before beginning treatment and deflates whenever the therapy is paused. The device can also be switched to Spanish mode when desired and has capabilities for other languages, as well.
Jurors were most impressed with the fact that they could operate the vest almost immediately. “The whole device is exquisitely simple to use,” says Wiklund. “My fellow judges and I were able to operate it immediately without needing respiratory therapy expertise or instructions. This suggests that patients and assistants, such as a parent, should have no trouble using the device.”
|
Euromed's SureSkin central catheter IV dressing uses a hydrocolloid adhesive that enables repositioning but can maintain adhesion for up to seven days. |
The SureSkin central catheter IV dressing from Euromed Inc. (Northvale, NJ) is designed to ensure secure yet gentle waterproof adhesion, with flexibility so that it conforms to surfaces on the body that are not flat. The dressing incorporates a hydrocolloid adhesive that is thin and flexible. It is designed to adhere up to seven days if necessary.
To design the product, Euromed approached several problems seen in many central catheter dressings. For example, most dressings have corners, are relatively flimsy, and use several picture frame–type release liners. These dressings can lift at the corners and the shape does not make it easy to apply to areas such as the neck. In addition, during application the adhesive may wrinkle or stick to itself, which can make the dressing unusable. “One of our employees has a wife who is a phlebotomist,” says Richard Lovell, director of marketing at Euromed. “She helped us identify some of the problems in typical dressings.”
To create the SureSkin, the design team developed an overlapped release liner that only has two pieces and allows application with gloves. The dressing is round and has a multidimensional border and a special tube outlet area. “We wanted to enforce the edge, but still enable it to be flexible,” says Lovell. The border provides stability to prevent the dressing from wrinkling beyond usability. And the tube outlet ensures a secure seal around the tube area.
The shape discourages lifting at the edges and promotes adhesion to curved body areas. “Intuitively, its round shape seems more appropriate for such a dressing than rectangular ones in terms of ensuring patient comfort and keeping the edges from peeling up,” says Wiklund.
The device is also transparent so that users can see where they are going to put it, explains Lovell.
“The SureSkin shows the advantages of materials integration into a convenient and effective device that is easy to apply and adheres effectively, but removes easily when necessary,” says Jackson.
Jurors also like the fact that nonprofessional caregivers can apply the dressing. “I like that it's caregiver inspired,” says juror William Schneeberger, a cardiothoracic surgeon at the University of Cincinnati Medical Center. “These types of products cost about $3 each and the applier usually has only one chance to get it right. The SureSkin is more forgiving. Even with a little fold in the adhesive, the product can still be used.”
Conclusion
Simplicity of design is often a deceptive phrase considering the amount of work that designers put into a product or package. But a device that harnesses fundamental shapes and technical elements will ultimately be easier to use, and therefore it is more likely to be adopted by the user or the patient.
Several manufacturers of this year's MDEA winners found the answers to simple design by talking to experts, by seeking alternatives to time-consuming or expensive practices, or by cutting costs on unnecessary features or technologies so they could put more emphasis on human factors. By striving for simplicity, these winners were able to convey the quality and innovation of their products.
Copyright ©2007 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like