Interoperability in Healthcare Tech Could Save $30 Billion
March 26, 2014
|
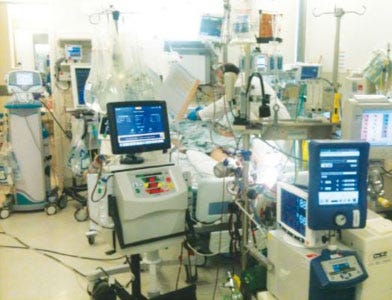
This is an actual photo of an intensive care unit room. There are more than 10 devices in this room and they are not seamlessly sharing information. "Does this look like smart healthcare?" asks Nick Valeriani, CEO, West Health. (Courtesy West Health) |
A white paper has been jointly released by the Gary and Mary West Health Institute and the Office of the National Coordinator for Health Information Technology (ONC) that describes the need for and tells stakeholders how to achieve an interoperable healthcare system.
The product of a February conference that drew more than 1700 to Washington, DC, the white paper, "Igniting an Interoperable Health Care System," tells the medical technology industry that they must "collaborate and partner to promote the development and adoption" of interoperability."
Interoperability, described as "the ability of systems to exchange information and to use the information that has been exchanged" in the conference materials, can help solve the healthcare crisis.
"Interoperability is a burning issue that impairs care delivery, and patients are waiting for it to be improved," said Nicholas Valeriani, CEO of San Diego, CA-based West Health. "Interoperability can enable a smarter healthcare delivery system and I encourage all stakeholders to recognize that the lack of interoperability is a crisis and to advocate for rapid change."
The 30-page white paper, which features a 10-page executive summary, provides guidance on how healthcare technology vendors, standards development organizations, hospitals, health systems and clinicians, regulatory agencies, and payors can all take action to drive interoperability. Even patients and the investor community have a role to play, the paper says.
And FDA is weighing in. Jeffrey Shuren, CDRH director, announced that FDA would soon be releasing a draft guidance on interoperability. The guidance will be non-binding, Shuren says, but FDA will make it easier for devices that meet interoperability standards to get clearance.
"FDA's role is to provide the regulatory environment to promote interoperability," Shuren wrote in the white paper. "The value of consensus standards is that we can say: If you conform with these standards, we're viewing you as having met the regulatory requirements. We are not telling you that you must conform, but if you do and we have adequate assurances, that's good enough for us....We're looking to provide the incentives to move toward that approach."
Shuren continued, "Interoperability is important for innovation. It truly gives us the opportunity to share and aggregate data between technologies and also to pull from them, to support the move to big data and improving healthcare. It can help enhance existing functionality, and it can help create new functionality. Interoperability is also important to the ability to provide care in different settings."
Executive editor of the white paper was Joseph Smith, MD, PhD, chief science and medical officer of the West Health Institute. Contributing editors include Doug Fridsma, MD, PhD, chief science officer & director, Office of Science & Technology, ONC; Mark Leahey, president and CEO, Medical Device Manufacturers Association (MDMA); and Bakul Patel, senior policy advisor to the Center Director, CDRH.
"ONC envisions an information-rich, consumer centered health care system that allows a patient's health information to follow them wherever they get their care," said Karen DeSalvo, MD, national coordinator for Health IT, in the press release announcing the white paper's publication. "We are working with patients, providers and others across health care and health IT to securely and safely free health information that will help to improve patient care and health, at lower costs."
Stephen Levy is a contributor to Qmed and MPMN.
About the Author(s)
You May Also Like