Hydrocolloid PSAs: New Formulation Strategies
June 1, 1999
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
An MD&DI June 1999 Column
New hydrocolloid compositions minimize skin reactions by eliminating the tackifying agents in adhesives integrated with styrene-isoprene-styrene thermoplastic elastomers.
Hydrocolloid pressure-sensitive adhesives (PSAs) were originally developed as oral-cavity bandages to aid in drug delivery to the gingiva. These adhesives are unique because they are inherently both adhesive and absorbent. They are useful as wound dressings because they can be applied directly to open wounds and can be secured on the surrounding intact skin. They are also useful in skin-barrier applications such as protecting the peristomal skin of ostomy patients. Hydrocolloid skin barriers can be divided into integrated and nonintegrated compositions. In this context, integrated refers to compositions that substantially retain their dimensional stability and form when saturated with wound exudate or other body fluids. Nonintegrated compositions include those that become a soft gel and amorphous as they are saturated with fluids.
Particularly in the area of wound dressings, a number of limitations have hindered the success of hydrocolloid adhesives. First, the absorption capacity of hydrocolloid dressings is normally insufficient to handle the large amount of exudate from some chronic wounds. Second, hydrocolloid compositions usually contain water-soluble absorbents. These produce the characteristic wet tack, but the soluble absorbents can leach out into the wound and be absorbed into the body. Third, hydrocolloid compositions are normally opaque, so healing cannot be assessed until the dressing is changed. Fourth, the continuous phases in many nonintegrated hydrocolloid adhesives contain low-molecular-weight elastomers such as polyisobutylene. In a nonintegrated hydrocolloid, the polyisobutylene is dispersed in the soft gel that remains in the wound after dressing removal, and it is possible for the elastomer's residues to get trapped in the cellular structure of chronic wounds as they heal. Although polyisobutylene is chemically saturated and is therefore inert, it has been suspected as the cause of abnormal foam cells (or giant cells) observed in the histology of healing dermal tissues. Indeed, it has been reported that residues of a synthetic binder were identified in tissue treated with a hydrocolloid dressing. The presence of binder had a negative effect on the healing rate of acute wounds in rats.1 It therefore became extremely important to eliminate leachable material from these otherwise very useful compositions.
Market research studies suggest that hydrocolloid adhesives will play an increasingly important role in preventing and treating chronic wounds, in addition to their continued use by ostomy patients as a barrier material. A recent report forecast that the Western European market for hydrocolloid and hydrogel wound dressings will grow at a compounded annual growth rate of 12.5% from 1995 to reach $383 million by 2002.
HYDROCOLLOIDS IN MEDICAL DEVICES: AN OVERVIEW
In the 1960s, the first hydrocolloid compositions, which were described in Squibb's basic patent, were inelastic and nonintegrated. They failed to maintain their dimensional stability, and they became amorphous when imbibed with wound fluid or other body fluids.2 A typical formulation described in Squibb's patent is the composition formed from low-molecular-weight polyisobutylene (40% by wt), pectin (20% by wt), sodium carboxymethyl cellulose (20% by wt), and gelatine (20% by wt). This formulation was used as a gingiva dressing and was also the basis of commercially successful skin-barrier and wound-care products. Unfortunately, when in contact with an exuding wound, such compositions form a soft gel that remains in the wound after the dressing is removed. The remaining gel must be irrigated from the wound, which is not only time-consuming for the caregiver but also painful for the patient.
Notwithstanding the drawbacks of these early formulations, however, the Squibb compositions are extremely gentle to intact skin. Several factors contribute to this. First, Squibb's compositions contain relatively few components so that, statistically, few skin reactions can be expected. Second, the ingredients are usually food components or additives and therefore have a documented history of contact with mucosal surfaces. Third, polyisobutylene contains a chemically saturated aliphatic carbon-carbon backbone, and therefore needs no stabilizer to reduce degradation often seen in rubbery materials that have unsaturated backbones. Fourth, the compositions apparently maintain optimum skin moisture by absorbing excess perspiration and reducing the amount of skin maceration normally associated with wearing a wound dressing for several days. Skin maceration leads to reduced mechanical strength of the skin, which in turn leads, on removal of the bandage, to increased damage to the healthy skin surrounding the wound. This is often termed mechanical irritation. Other compositions have integrated the continuous phase at the expense of the skin-friendly characteristics displayed by the Squibb compositions.
Hydrocolloid Compositions with an Integrated Continuous Phase. Lack of integrity was a serious drawback in the early Squibb formulas, particularly as the basis for hydrocolloid dressings. After much research was conducted to overcome the deficiency, Coloplast patented improved integrated hydrocolloid compositions.3 These formulations provided a sealing material for ostomy use that consisted of either a hydrocolloid dispersed in a continuous phase of styrene-isoprene-styrene (S-I-S) copolymer or some other thermoplastic elastomer, such as an ethylene-propylene copolymer. Also present in the formulas are a hydrocarbon tackifier and an optional oil extender and antioxidant. Because the resulting material is elastomeric and flexible, the bandages made from it are comfortable and adhere well to skin. The S-I-S block copolymer, which provides pseudo-cross-linking of the styrene domains, enables the composition to be integrated. At room temperature, the S-I-S block copolymer forms physical cross-links within the continuous phase. Such cross-links are formed because the polystyrene segments within the copolymer are incompatible with the polyisoprene segments. At room temperature, they associate to glassy domains that act as physical cross-links to form a three-dimensional lattice. However, a lower absorption level is obtained because Coloplast's absorbent hydrocolloid components are normally integrated at a lower concentration in the final formulation than they are in the basic Squibb product. The absorption rate is also slower, because the integrated nature of the composition makes the lower level of chemical hydrocolloid components accessible to the body fluid even more slowly.
Researchers at Hollister also recognized the shortcomings of barriers and dressings based solely upon a continuous phase of polyisobutylene.4,5 Hollister's two patents discuss barriers and dressings based on an integrated formulation containing a continuous phase composed of a blend of high vinyl acetate containing ethylene-vinyl acetate (EVA) copolymer (51% VA and 49% ethylene by wt) and low-molecular-weight polyisobutylene, in which a discontinuous phase containing a blend of a superabsorbent material, pectin, and sodium carboxymethyl cellulose is dispersed. The function of the EVA copolymer is to cross-link in the presence of ionizing radiation, such as gamma radiation at a dosage of 25 kGy, which would be used to sterilize dressings made from these compositions. The integrated network is formed from the EVA polymer by cross-linking during irradiation. The problem with this type of system is that the dose from such a sterilization process varies widely in practice. A company offering services for the sterilization of medical devices to a nominal dose of 25 kGy would typically specify a dose within the range of 25–35 kGy, for example. Some dressings would receive close to the lower amount, whereas some would actually be exposed to the higher amount. Such variation leads to variable cross-link density within dressings of even the same production batch, which in turn leads to inconsistent performance in terms of rate and fluid-absorption capacity.
The 3M Company also prepared radiation-sensitive hydrocolloid compositions based on hydrophobic unsaturated aliphatic homopolymers such as polyisoprene and polybutadiene.6 Polyisobutylene polymers provide the necessary tack and pressure-sensitive adhesion, which ensures that the continuous phase is filled with either soluble or insoluble absorbent hydrocolloids.
Further work by Squibb describes integrated hydrocolloid adhesives modified by diluting the amount of S-I-S block copolymer in the composition.7 The patent describes a medical-grade pressure-sensitive adhesive composition comprising a mixture of one or more polyisobutylenes or blends of polyisobutylenes and butyl rubber, one or more styrene radial or block copolymers, a tackifier, mineral oil, and one or more water-soluble or swellable hydrocolloid gums. The polyisobutylenes, butyl rubber, mineral oil, and tackifier predominantly modify and plasticize the isoprene segment of the block or radial copolymer. The mineral oil is said to provide increased extensibility and aggressiveness to the adhesive. It is believed that this patent forms the basis of the DuoDerm and Signa Dress, two commercially available hydrocolloid dressing products. The saline absorption rate with these compositions, however, is very slow and not very reproducible. Moreover, the absorption level is significantly lower than the absorption levels available with polyisobutylene-based compositions.
The patents described above provide the basis for commercially available hydrocolloid dressings and skin barriers. In all of the later formulations, modifications are made to the continuous phase to achieve integrated compositions. In each case, the integrated continuous phase is achieved only at the expense of another beneficial property found in Squibb's original nonintegrated composition. Moreover, because integrated compositions have many components—in particular the tackifying resin and stabilizers—the dressings made from these modified hydrocolloid materials tend to elicit more complaints about irritation than the original polyisobutylene-based product.8
Hydrocolloid Compositions with an Integrated Discontinuous Phase. The formulations cited above seek to improve the integrity of hydrocolloid compositions by modifying the continuous phase. A different approach was taken in another patent, in which Squibb describes incorporating into the discontinuous phase a cohesive strengthening agent such as natural or synthetic fibrous materials and other insoluble absorbent polymers.9 The cohesive strengthening agent decreases the tendency toward tearing and the tendency toward erosion and disintegration by biological fluids. The swelling of the hydrocolloids, therefore, is said to be controlled.
The 3M Company has patented adhesives that contain polycationic hydrocolloids—preferably water soluble—such as a chitosan salt and dextran mixed with polyanionic and neutral hydrocolloids such as pectin and gelatine, respectively.10 These compositions are said to possess high integrity. The continuous phases described in both of these Squibb and 3M patents, however, are conventional in that they are composed of a mixture of low-molecular-weight polyisobutylene and high-molecular-weight rubber. Squibb uses high-molecular-weight butyl rubber, and 3M uses high-molecular-weight polyisobutylene rubber.
In summary, existing compositions integrate hydrocolloid using one of three approaches: thermoplastic elastomers, usually S-I-S elastomers, together with alicyclic or aromatic tackifying resins; radiation-sensitive elastomers that form a cross-linked matrix during sterilization; or ionically charged absorbents or cohesive strengthening agents in the hydrocolloid to integrate the adhesive matrix.
Conventional tackifying resins are irritants and allergens and therefore have no place in contact with chronic wounds. The resulting adhesives are aggressive to intact skin. Note that thermoplastic elastomers do not themselves possess adhesive properties. To generate pressure-sensitive characteristics, a tackifier or a plasticizer in combination with a thermoplastic elastomer must be added.
Cross-linking of the matrix by radiation dosing appears attractive because dressings must be sterilized, and gamma radiation is the usual method for sterilizing these products. However, this approach is also unsatisfactory because the dose varies greatly in practice. This variation causes a disparity in cross-link density even within the same production batch. Moreover, when used as ostomy barriers, hydrocolloid adhesives generally do not need to be sterilized, but they may still need to be integrated to maintain durability in the presence of urine. Therefore, using radiation only to achieve integration becomes uneconomic if it can be achieved by other means.
Charged absorbents and cohesive strengthening agents do not provide total integration. If they are used as the only integrating mechanism, these absorbents and agents require the presence of plasticizers or low-molecular-weight polyisobutylene. These elements can leach into a wound and produce giant cell formations or other undesirable effects.
NEW DEVELOPMENTS IN HYDROCOLLOID RESEARCH
The goal of new research was to develop integrated hydrocolloids suitable for wound contact that would not contain extractables that could be absorbed by the wound. Other compositions use mostly thermoplastic elastomers to integrate the continuous phase, and tackifying agents are often added to the elastomer to provide tack and adhesion to the final formulation. This research sought to develop integrated hydrocolloid formulations that eliminate tackifiers in particular, but also polyisobutylene, while maintaining manageable processing characteristics.

Figure 1. Representation of integrated continuous phase of existing hydrocolloid systems.
It is useful to depict the three-dimensional structure likely to result from using the approaches discussed above to integrate hydrocolloids. The schematic in Figure 1 shows such structures for cases where S-I-S is the integrating polymer. In this case, the plasticizing entity is not permanently bound within the matrix. A similar situation occurs when other integrating polymers, such as radiation-cross-linked polybutadiene or polyisoprene, are used.6 When charged absorbents and cohesive strengthening agents are used as the integrating medium, the plasticizing polymers are still free to migrate from the matrix.
In Figure 1, the thermoplastic elastomer is in a pseudo-cross-linked state because the association of the polystyrene portions of the polymer form glassy islands or domains. Such association is generally accepted in this type of polymer system and is the thermodynamically preferred state at ambient temperatures. The glassy domains reinforce the structure and act as the cross-linking junctions in the three-dimensional matrix. Although other plasticizers or polymer molecules may be compatible with elements of the three-dimensional structure, they will not be firmly held by it and will be free to migrate out.
New materials that have recently become available commercially are low-viscosity plasticizers marketed as nonmigrating plasticizing additives for styrene block copolymer thermoplastic elastomers. These materials appeared to be ideal because they offer the potential for formulating a hydrocolloid that has a continuous phase with no extractable components.
The new fluid-absorbing adhesives comprise a continuous phase consisting of a solid, physically cross-linked thermoplastic elastomer, such as styrene-olefin-styrene or styrene-alkane-styrene copolymer, and a low-viscosity component that is substantially resin free. The continuous phase provides dry tack to ensure initial adhesion to dry skin. A discontinuous phase consisting substantially of absorbent polymer is dispersed within the continuous phase. The new formulation also uses an S-I-S thermoplastic elastomer to integrate the hydrocolloid matrix, but it uses no conventional tackifier. A compatible low-molecular-weight liquid rubber, preferably with the same or similar chemical makeup as the thermoplastic elastomer, performs the tackifying function. The preferred low-molecular-weight liquid rubber is a styrene and isoprene block copolymer, which contains polystyrene residues. In the adhesive, the polystyrene residues become physically associated to the elastomer through the glassy polystyrene domains. Therefore, the tackifying plasticizer is permanently associated with the rubber so it cannot leach out into the wound. This is a new approach to hydrocolloid integration, and the products are chemically unique.
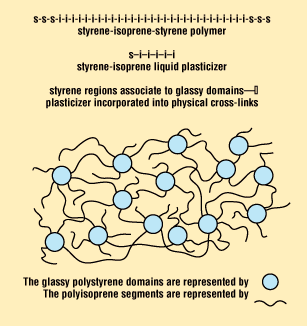
Figure 2. Representation of the cross-linked continuous phase of the new hydrocolloid adhesives.
Figure 2 shows a schematic representation of the cross-linked continuous phase of new patent-pending hydrocolloid adhesives. In this figure, the short lengths of polyisoprene block that form part of the liquid styrene-isoprene (S-I) rubber plasticizer are shown as free-ended lines, whereas the polyisoprene segments of the S-I-S thermoplastic elastomer are depicted as a line joining two glassy polystyrene domains. Both polymer types—the low-molecular-weight S-I plasticizer and the higher-molecular- weight S-I-S elastomer—are linked through their common polystyrene segments. Within the newly formed three-dimensional network, the absorbent macromolecules are evidently tightly held.
EXPERIMENTAL PROCEDURES
Formulations were made in a variable-speed Z-blade mixer with extruder discharge and a heating capability to about 220ºC. The adhesive compositions were prepared using the following steps and conditions.
The solid rubber, such as a styrene-olefin-styrene copolymer, and the low-viscosity S-I component are blended together in a suitable mixer, normally a sigma blade mixer with an extruder discharge. The mixer is heated to about 170ºC. A nitrogen flow of about 60 ml/sec through the mixer reduces the possibility of oxidative degradation of the rubber during processing. About 1% parts per hundred parts rubber (phr) of a suitable stabilizer (say Irganox 1010 from Ciba-Geigy) can be added at this stage. The plasticizer is added proportionally to allow it to blend with the soft solid rubber. When all of the plasticizer has been added, the mixture produces a pourable, tacky, intermediate adhesive. The mixer blades are stopped, the direction of the screw is reversed, and the intermediate adhesive is removed from the mixer. It is poured into suitably release-coated containers and allowed to cool. The mixer is stabilized at 90ºC, and the powdery ingredients are added to the mixer with other optional ingredients and are blended in. After mixing at 90ºC for 20—30 min, the mixer temperature is raised to 105ºC, and the ingredients of the continuous phase—the intermediate adhesive—can then be added. The fully mixed mass is removed from the mixer, extruded or pressed to the desired thickness, and then laminated to suitable substrates.
The following materials were used in these investigations:
Tackifier: Regalite 91, Hercules Chemical Co.
S-I-S triblock: Vector 4111, Exxon Chemical Co.
S-I-S diblock and triblock: Kraton KD-1161N, Shell Chemical Co.
S-I plasticizer: LVSI-101, Shell Chemical Co.
Stabilizer: Irganox 1010, Ciba Products.
Insoluble absorbent: Aqualon A500, Hercules Chemical Co.
Soluble absorbent: Pectin USP100, Hercules Chemical Co.
Sodium carboxymethyl cellulose: Blanose, Hercules Chemical Co.
Polyisobutylene: Vistanex LMMH, Exxon Chemical Co.
TEST PROCEDURES
The following test procedures were used to determine the final formulation: reverse tack, 90°-peel adhesion, static shear, static absorption, cold flow, and integrity.
Reverse Tack. This test determines the maximum force necessary to remove a standard polyester strip from a hydrocolloid surface after it has been brought into contact with the hydrocolloid with no external force.
To conduct this test, make the test panel self-adhesive using double-coated tape. Laminate the hydrocolloid adhesive on the test panel. Place the test panel with hydrocolloid in the lower clamp of a tensile testing machine. Program the tensile tester. Place a polyester test strip with a thickness of 125 µm (5 mil) and dimensions of 21 X 2.54 cm in the upper clamp, making sure that a 15-cm length of polyester is under the clamp (loop). Remove the release liner from the hydrocolloid and start the measurement. Record the maximum force necessary to remove the polyester strip from the hydrocolloid surface.
90º-Peel Adhesion of Hydrocolloid Adhesives on Stainless Steel. The peel adhesion on stainless steel measures the average force to remove a hydrocolloid adhesive—laminated under specified conditions on a stainless-steel panel—from the stainless-steel panel at a constant speed and at a 90° angle.
Before conducting the test, clean the stainless-steel panel with solvent. Cut a 25.4-mm-wide hydrocolloid sample and reinforce it with tape. Laminate a paper strip at one end of the hydrocolloid sample using an overlap of about 1 cm. Remove the liner from the hydrocolloid sample, and laminate the sample on the stainless-steel panel with a 45-g roller at 150 cm/min. Allow the sample to dwell for 1 minute. Place the paper strip in the upper clamp and the stainless-steel panel on the lower clamp. The angle between the peel direction and stainless-steel panel must be 90º. Start the measurement using a crosshead speed of 300 mm/min. The angle must be kept at 90º until the measurement is complete. The 90º-peel adhesion is the average force necessary to remove the hydrocolloid strip from the stainless-steel panel.
Static Shear of Hydrocolloid Adhesives. This test determines the time necessary to remove a hydrocolloid adhesive—laminated on a stainless-steel panel under specified conditions—from the test panel under the influence of a specified weight.
This test requires that the hydrocolloid samples be conditioned at 23º ± 1ºC and 50% ± 2% relative humidity for 24 hours. Clean the stainless-steel shear panel with solvent. Cut a hydrocolloid strip 25.4 mm wide and 50 mm long. Reinforce the hydrocolloid strip with tape. Laminate the hydrocolloid strip onto the test panel using an overlap surface of 1 sq in. Protect the free hydrocolloid with a release liner. Place a 500-g weight on the laminate for 1 hour. Reinforce the free hydrocolloid adhesive zone with plastic and then perforate it. Place the test panel with hydrocolloid on the shear bar using a 500-g shear weight. Zero the clock, and note the time when the sample falls off under
the weight.
Static Absorption of Hydrocolloids. This test determines the amount of fluid uptake into a known surface area of hydrocolloid adhesive.
It is important to laminate the release liner to the upper flange of a standard moisture vapor transmission cup with double-coated tape. This provides the contact zone for the hydrocolloid. Fill the cup with a 30-ml NaCl solution (0.9% by wt). Cut a hydrocolloid sample about the same size as the diameter of the outer cup. Weigh the sample (W1). Laminate the sample to the cup to ensure that the seal between the sample and the cup is watertight. Turn the cup upside down and put it in the oven at 37ºC for 24 hours. Remove the cup from the oven and allow it to cool. Remove the hydrocolloid from the cup and reweigh it (W2). Calculate the water fluid absorption (g/m2/24 hr) using the formula:
abs = (W2 – W1) / A
where A is the area in square meters of the hydrocolloid in contact with salt solution.
Determination of Cold Flow. To calculate cold flow, the flow of the hydrocolloid is measured under influence of a specified pressure and after a specified time. This test requires that hydrocolloid samples be conditioned at 23º ± 1ºC and 50º ± 2% relative humidity for 24 hours. Cut five samples of hydrocolloid using a 35-mm circular die-cutter. Put a silicone paper on top of a glass plate, arranging the samples on the paper so that pressure will be distributed equally. Measure the diameter of each sample with calipers, marking the measurement. Put a plastic disk on each sample. Put another silicone paper, two glass plates, and a 10-kg weight over the construction. (This measurement can also be taken by placing the samples with the disks and the 10-kg weight in a 40°C oven.) After 24 hours, measure the diameter of the samples where they are marked. Calculate the percentage increase of the diameter. The cold flow is the average percentage increase of diameter after a 24-hour exposure to 10 kg for five samples. Record both the average percentage increase in diameter and the test temperature.
Determination of the Integrity of Hydrocolloids. The integrity of a hydrocolloid is defined as its ability to resist breakdown by biological fluids. This test measures the percentage weight of hydrocolloid adhesive retained after exposure to saline under specified conditions.
The hydrocolloid samples must be conditioned at 23º ± 1ºC and 50% ± 2% relative humidity for 24 hours. Cut circular samples 2.54 cm in diameter from the hydrocolloid sheet. Weigh and record the samples (Wi). Place each sample in a bottle with 50 ml of aqueous saline (0.9% by wt). Cap the bottles and agitate on the bottle shaker at maximum speed for 18 hours. Remove the sample, and place it in a circulating air oven at 50oC and 50% relative humidity until dry. This process takes about 24 hours. Weigh and record the sample (Wf). The integrity value of the sample is calculated using the following equation:

RESULTS
Table I shows the composition of four examples prepared in accordance with the general directions described earlier. Some formulations were made without polyisobutylene. The results showed that a wide range of formulations are possible and that ratios of plasticizer to rubber can vary over a range of more than tenfold to yield formulations with useful properties. The examples in Table I were evaluated using the test procedures described. The results are shown in Table II.
Table I. Four formulations composed of various materials. All were tested, yielding the results in Table II. |
You May Also Like


