Eating Disorder Community: New Weight-Loss Device Is ‘Dangerous’
July 28, 2016
The Academy for Eating Disorders called the AspireAssist device "truly disturbing" and is asking FDA to revoke its approval.
Nancy Crotti
Updated July 29, 2016
Qmed readers recently told us they find Aspire Bariatrics' recently approved stomach-draining device "scary." Apparently, the Academy for Eating Disorders agrees.
The group's president plans to submit a letter to FDA asking the agency to revoke its recent approval of the AspireAssist, an obesity treatment device that uses a surgically placed tube to drain a portion of the stomach contents after every meal.
Members of the Academy for Eating Disorders--psychiatrists and psychologists who treat people with anorexia, bulimia, and binge-eating disorder, as well as eating-disorder researchers and advocates--consider the device "dangerous," according to the letter. It was cosigned by nine other eating-disorder advocacy groups.
The groups are not criticizing FDA, said Elissa Myers, the academy's executive director. Their members believe the agency did not have enough evidence to approve it, and fear that people with undiagnosed eating disorders could obtain the device, perpetuate their illness, and even die.
Their letter calls the AspireAssist "a truly disturbing incidence of technology serving to perpetuate pathology."
Aspire Bariatrics CEO Katherine Crothall countered that patients who received the ApsireAssist were monitored for eating disorders throughout clinical trials.
"There was no evidence of patients developing adverse eating behaviors," she told Qmed by email. "Successful use of the device requires thorough chewing, otherwise the device will clog; hence patients cannot binge-eat and then 'purge' with device. Data from multiple studies indicate that patients improve their eating behaviors with the device with thorough chewing, greater mindfulness about eating, and reduced caloric consumption."
Crothall also reiterated the need for the device.
"With over 25 million Americans suffering from morbid obesity and a paucity of options to treat obesity, it is sad that people who know nothing about the device are trying to remove it from the market, one of the few viable options to treat obesity," she wrote.
FDA did not immediately respond to requests for comment.
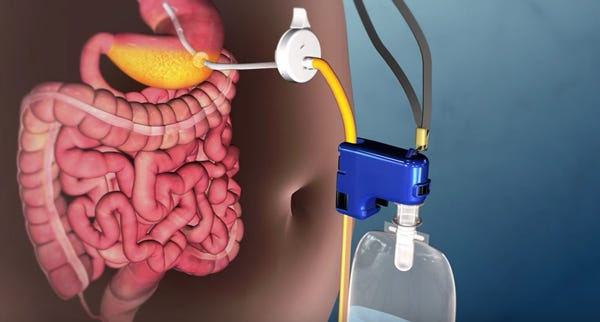
To place the device, surgeons insert a tube in the stomach with an endoscope via a small incision in the abdomen, according to a statement FDA released in announcing its approval of AspireAssist. A disk-shaped port valve that lies outside the body, flush against the skin of the abdomen, is connected to the tube and remains in place. Approximately 20-30 minutes after meal consumption, the patient attaches the device's external connector and tubing to the port valve, opens the valve and drains the contents. Once opened, it takes approximately five to 10 minutes to drain food matter through the tube and into the toilet. The device removes approximately 30 percent of the calories consumed, the agency said.
FDA said it issued its approval after reviewing results from a clinical trial of 111 patients treated with AspireAssist, nutrition and exercise counseling, and 60 control patients who received only the counseling therapy. After one year, patients using AspireAssist lost an average of 12.1% of their total body weight compared to 3.6% for the control patients, the agency noted.
Clinical trial results also suggested that both patient groups had small improvements in conditions often associated with obesity, such as diabetes, hypertension and quality of life, FDA said, adding that the lifestyle therapy may have led to these results.
The advocacy groups wished they had had the opportunity to address FDA on "the unintended consequences of this approval," according to Myers.
"This will likely prove to be yet another in a long list of misguided, unsuccessful, and dangerous products for losing weight," wrote academy president Eva Trujillo,MD. "For individuals of any size and especially those in larger bodies who may be seeking remedies to reduce size or restrict food intake, this device can compound unhealthy, potentially life-threatening eating disorder-related behaviors,"
In announcing its decision to approve AspireAssist, FDA saidthe device is not intended for use on people with eating disorders. It was approved for patients aged 22 and older who are obese, with a body mass indexof 35 to 55, and who have failed to achieve and maintain weight loss through non-surgical weight-loss therapy.
A physician who prescribes the device may not know if the patient has an eating disorder, because it's not possible to determine if someone has an undiagnosed eating disorder just by looking them, according to Myers.
"If you are somebody with anorexia you may not be presenting as severely underweight," she explained. "In fact, you may be presenting as slightly overweight."
Nancy Crotti is a contributor to Qmed.
About the Author(s)
You May Also Like


