Did Olympus Fail to Report Deadly Scope Problems?
December 22, 2015
An Olympus employee in the Netherlands found contamination in a TJF-Q180V duodenoscope as early as 2012, but the Japanese multinational for years did not raise alarms in the U.S., according to an LA Times investigation.
|
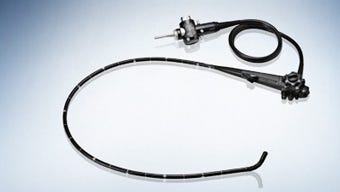
Olympus' TJF-Q180V duodenoscope, as shown on the company's website |
Qmed Staff
Olympus failed for years to alert U.S. hospitals that its TJF-Q180V duodenoscope had been linked to superbug outbreaks, even as the deaths mounted and more people were put at risk, according to a Los Angeles Times report based on "interviews with dozens of hospital officials, doctors, regulators, and former Olympus employees."
The story, published December 19, says an Olympus technician based in the Dutch town of Zoeterwoude discovered a film of bacteria inside a TJF-Q180V duodenoscope back in 2012. The scope was suspected of causing an outbreak at a hospital in the Netherlands that sickened 22 patients, and in fact, the Olympus technician found the rubber ring meant to keep bacteria out was cracked and worn. An independent investigator hired by Olympus and the hospital recommended a worldwide investigation, and a recall if there were more problems.
Instead, Olympus was mum about the TJF-Q180V problems in the United States, even as it issued "important safety advice" in Europe in 2013, and another European safety alert about tainted scopes in 2014, according to the LA Times.
Over the three years since the discovery of problems in the Netherlands, 21 people died and a dozens more were sickened from scope-related infections in Los Angeles, Pittsburgh, and Seattle, the Times reports. During each outbreak, Olympus officials blamed the hospitals' cleaning procedures, and did not tell hospital officials that there were scope-related outbreaks elsewhere, according to the paper.
A company statement an Olympus spokesman shared with Qmed does not directly address the claims in the LA Times report, but did express sympathy for people infected in the superbug outbreaks and their families.
"The current issue associated with duodenoscopes is receiving the highest level of attention at Olympus, and we remain committed to understanding and addressing the potential root causes," the Olympus statement says.
Olympus is the major U.S. seller of duodenoscopes, which are threaded down through the digestive tract and into the small intestine. Fujifilm, and Pentax's Hoya Corp. also sell models. Duodenoscopes provide the least invasive way of draining fluids from pancreatic and biliary ducts blocked by cancerous tumors, gallstones, or other conditions.
"Recently, more than 600,000 duodenoscope procedures have been performed safely in the U.S. annually, many of which have been critical or life-saving," Olympus said in its statement.
But by 2015, FDA was warning that the duodenoscopes' movable "elevator" mechanism, located at the tip, might improve efficiency and effectiveness, but has proven challenging to disinfect.
A slew of lawsuits against Olympus claim that was especially true with the TJF-Q180V, which the LA Times says was introduced in 2010 to compete against scopes from Fujifilm and Pentax that were easier to clean because a crucial part was sealed off to keep bacteria out.
The U.S. Justice Department is reportedly investigating how Olympus handled the outbreaks.
It wasn't until early 2015 that enough news had been generated from the outbreaks to spark warnings from FDA, which divulged that the TJF-Q180V had initially made it to market without a 510(k) clearance. By March, FDA was reporting that Olympus had "issued new, validated manual reprocessing instructions for the TJF-Q180V" as part of the review of a 510(k) application for the device, which FDA allowed Olympus to continue marketing while the application was under review.
FDA has issued warnings to Olympus, Fujifilm, and Pentax, ordering the three manufacturers to conduct postmarket surveillance studies, and elaborated on cleaning options for the scopes. An FDA advisory panel in May recommended costly sterilization before reuse.
By the fall, FDA was expanding its warnings to include flexible bronchoscopes made by Olympus and other companies.
Learn more about cutting-edge medical devices at MD&M West, February 9-11 at the Anaheim Convention Center in Anaheim, CA. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like