New Artificial Proteins Could Make Better Scaffolds for Tissue Engineering
Originally Published MDDI May 2004R&D DIGESTErik Swain
May 1, 2004
Originally Published MDDI May 2004
R&D DIGEST
|
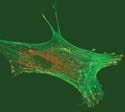
The end modules attract each other and form bundles, encouraging the growth of selected cell types. |
A new class of artificial proteins could help reduce development time on tissue-engineering projects. Researchers at The Johns Hopkins University (Baltimore) have produced a self-assembling protein gel, or hydrogel, that can encourage the growth of selected cell types. Hydrogels are macromolecular networks immersed in water that provide a framework, or scaffold, on which to grow cells.
“We're trying to give an important new tool to tissue engineers to help them do their work more quickly and efficiently,” says lead researcher James L. Harden, PhD, an assistant professor in the university's Department of Chemical and Biomolecular Engineering. “We're the first to produce a self-assembling protein gel that can present several different biological signals to stimulate the growth of cells. We feel that our system will make it easier for tissue engineers to systematically investigate the response of a particular cell type or a combination of cell types to different combinations of known cell-binding and -signaling ligands in a scaffold. This provides a simple way to optimize tissue-engineering scaffolds for particular applications.”
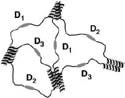
The new hydrogel is made by mixing specially designed modular proteins in a buffered water solution. Each has a bioactive sequence and is flanked by helical associating modules on each end. The end modules come in three distinct types, which attract each other and form bundles. The bundling leads to the formation of a network structure of proteins. A scaffold can be assembled spontaneously when the protein elements are added to the solutions. Certain biological signals are needed to form different types of tissue, which occurs via adhesion, proliferation, or differentiation.
|
The hydrogel, which is made by mixing specifically designed molecular proteins in a buffered water solution, can lead to the formation of a network structure of proteins. |
“Our hypothesis was that these gels would support cell attachment and proliferation if the center soluble spacer block contained appropriate cell binding and/or signaling ligands,” said Harden. “The particular ligands involved will depend on the choice of cell type. We have verified our hypothesis for several cell types.”
Harden reported his findings at the American Chemical Society's national meeting on March 28, 2004, in Anaheim, CA.
Specific applications have not yet been pinpointed, but Harden notes that the system is “of general utility for tissue engineering. The hydrogels . . . may be used as stand-alone scaffolds for cell and tissue cultures, or as biofunctional coatings for a traditional porous polymeric scaffold material, such as PLGA polymer foams.”
He said, however, that because they are physically cross-linked, rather than chemically cross-linked like synthetic polymer gels, they are “softer” and in some applications may be better suited as coatings than as standalone scaffolds. He also noted that the materials have not yet been characterized for biocompatibility and immunogenicity. Their fitness for use inside the body will not be known until that happens.
The goal is to provide a large combinatorial library of these hydrogels, from which tissue engineers could draw. “We want to let the end user mix and match the modules to produce different types of hydrogels for selected cell- and tissue-engineering projects,” Harden says. This means that because the hydrogels form spontaneously, tissue engineers would not need to go through the complex process of preparing a hydrogel for each specific application. Also, because more than one protein signaling segment can be included in a hydrogel mix, a tissue engineer can send multiple signals to the target cells. That means one tissue could see spontaneous growth of several types of cells.
Harden's research team also includes Lixin Mi, PhD, a postdoctoral researcher at the National Institutes of Health, and Stephen Fischer, a doctoral student in Harden's department. The research was supported by a grant from NASA's Program on Human Exploration and Development of Space.
Copyright ©2004 Medical Device & Diagnostic Industry
You May Also Like