Babson Unlocks Critical Milestone Ahead of Commercialization
Babson Diagnostics' strategic partner, BD, has secured FDA clearance for the BD MiniDraw capillary blood collection system.
December 11, 2023

The company that aims to succeed where Theranos failed just achieved a critical milestone ahead of bringing its BetterWay blood testing service to market next year.
FDA recently cleared the BD MiniDraw capillary blood collection device from Babson Diagnostics' strategic partner, BD. The BD MiniDraw is a key component of Babson's BetterWay blood testing, a first-of-its-kind offering intended to combine the convenience of capillary blood collection with the quality of laboratory testing.
According to Austin, TX-based Babson Diagnostics, BetterWay will reimagine the entire blood testing process, using only a pea-sized amount of blood collected from a fingertip and delivering results equivalent to traditional blood testing methods. The BetterWay service is meant to enable blood testing by non-phlebotomists in convenient locations without sacrificing quality, accuracy, or the types of tests that are possible.
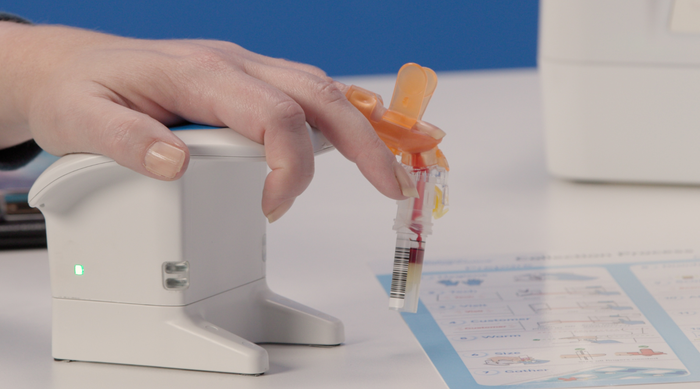
Person getting blood testing using the BD MiniDraw blood collection system at a retail pharmacy location. IMAGE COURTESY OF BABSON DIAGNOSTICS
“BetterWay is the blood testing service that consumers and healthcare professionals have been waiting for,” said Babson CEO David Stein. “Blood testing has remained largely unchanged over the past 70 years, yet it is a critical component of the care journey. BetterWay will help address the ongoing shortage of healthcare professionals as well as the increasing need to expand access and reduce healthcare costs."
As Stein has told MD+DI previously, the company put quite a bit of thought into designing its blood testing technology for primary care and family medicine, supporting annual exams, chronic condition management, and screenings. The launch menu is anticipated to offer the most frequently ordered blood tests and use existing CPT codes to enable easy clinician-directed orders and processing by payers.
With BD MiniDraw, BD will be a key supplier to Babson, and Babson will serve as a distributor for BD.
The Science Behind BetterWay Blood Testing
Bringing blood testing services to more locations requires expanding the pool of professionals who can collect blood. The BetterWay blood testing service is designed for healthcare settings without a trained phlebotomist, such as retail pharmacies, physician offices, urgent care centers, and skilled nursing facilities. Healthcare workers can be trained to perform the BetterWay service and to collect a high-quality amount of blood from a finger using BD MiniDraw.
The sample preparation steps that occur between blood collection and arrival at the laboratory—labeling, mixing, centrifugation, storage, and transportation—are often the source of sample quality issues. Babson’s sample preparation device is expected to automate pre-analytic processes and maintain optimal sample conditions. It is designed to reduce human interaction and error while maximizing quality. The sample preparation device makes metal storage boxes a thing of the past and seamlessly integrates into pharmacy operations.
Babson’s purpose-built, high-throughput lab uses market-leading analyzers adapted to produce high-quality results from capillary samples.
To date, Babson has validated the technology in its ecosystem by conducting more than 30 IRB-approved clinical studies—many with local and national retail pharmacy teams— involving thousands of subjects and more than 700,000 tests.
“BetterWay unites easy capillary blood collection and automated sample handling with the economies of scale and high quality of laboratory testing,” said Eric Olson, Babson's founder and chief operating officer. “As a science-first company, we’ve taken a systematic approach to accomplish what others have only talked about. Now we are poised to launch a true diagnostic blood-testing alternative, meeting the stringent requirements of today’s clinicians and the expectations of the modern consumer.”
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
