Ablative Solutions revealed data from the Peregrine Post-Market Study during the 2019 Cardiovascular Research Technologies meeting in Washington D.C. recently.
March 8, 2019
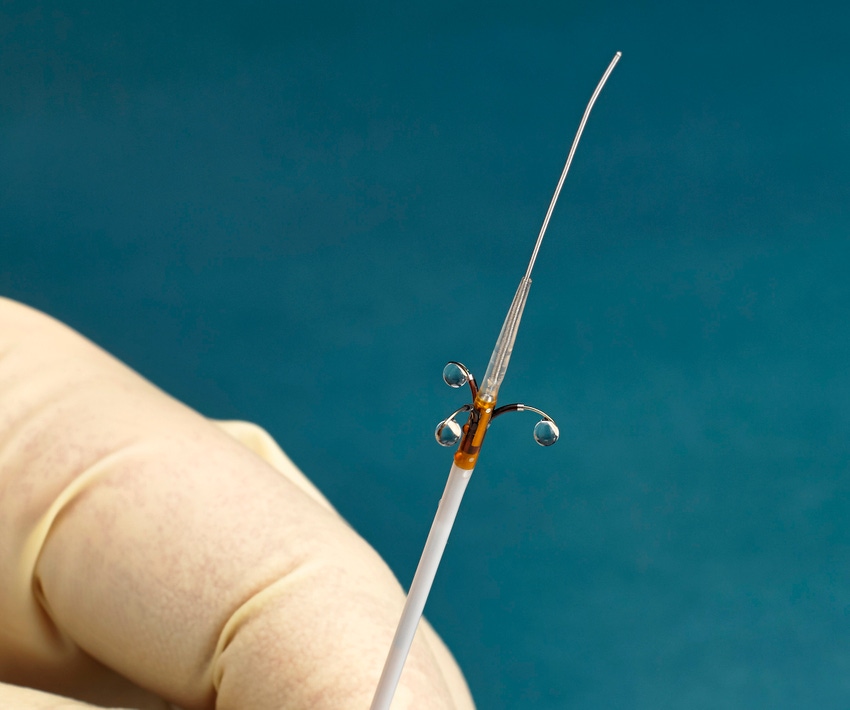
Ablative Solutions just made a significant contribution to the resurgence of renal denervation procedures for the treatment of uncontrolled hypertension. The Kalamazoo, MI-based company added to the treatment’s resurgence narrative by unveiling compelling data from the Peregrine Post-Market Clinical Trial.
The data reveal was during a late-breaking session at the 2019 Cardiovascular Research Technologies meeting in Washington D.C. last week.
The Post-Market Study is a European multicenter open-label trial that evaluated the safety and performance of the Peregrine System Infusion Catheter, which has CE mark. The device used alcohol as a neurolytic agent in 45 patients with systemic hypertension.
In the study, the efficacy endpoint was met, with a reduction in mean systolic 24-hour ambulatory blood pressure of 11mmHg <(± 14 mm Hg, p<0.001) at six-month follow-up. Additionally, the average reduction in systolic office blood pressure was 18 mmHg (± 21 mmHg, p<0.001). Antihypertensive medications were unchanged in 73% and reduced in 23% of patients at six months.
The study demonstrated 100% procedural success, and the safety endpoint was met in 96% of patients. Two patients had major adverse events of peri-procedural access site pseudoaneurysms, with major bleeding in one patient. There were no deaths or instances of myocardial infarction (MI), stroke, or transient ischemic attack.
“These promising results met the study’s safety and efficacy endpoints, confirming a safe and effective reduction in blood pressure by means of alcohol-mediated renal denervation, with 71% of patients responding,” Nicole Haratani, executive vice president of global clinical, regulatory affairs and quality at Ablative Solutions, said in a release.
Renal denervation for uncontrolled hypertension was once thought of as one of the hottest procedures with promise in medtech. But the narrative quickly changed for these procedures in 2014, when Medtronic failed to meet its primary endpoints in the SYMPLICITY HTN-3 trial.
Companies started abandoning the thought of the procedures and even Medtronic shelved its plans for a time. However, in April of 2018, the Dublin-based company reignited its efforts to get approval for the therapy when it announced it had received a nod from FDA for a pivotal trial to evaluate the Symplicity Renal Denervation System.
Ablative Solutions has been making strides and proved in January that venture capital investors were still very much interested in renal denervation for uncontrolled hypertension. The company was able to raise $77 million for its series D round. The round was led by new investor Gilde Healthcare and co-led by existing investor BioStar Ventures and an undisclosed new strategic corporate investor. Existing investors including Michigan Accelerator Fund, Novus Biotechnology and other individual investors also participated in the Series D funding round.
About the Author(s)
You May Also Like




