Close-up of a person wearing a tie and boxing gloves with a document in front of them that has Win/Lose checkboxes with a checkmark for "Win"
Implants
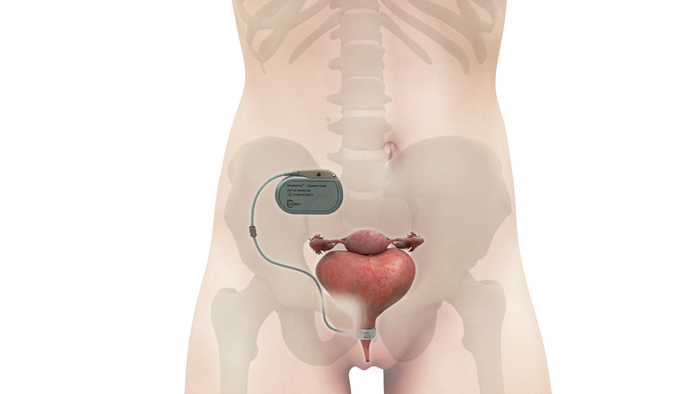
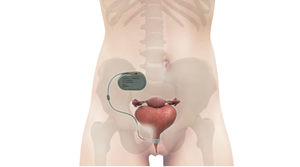
Medtronic Won't Back Down but Axonics Stands Its GroundMedtronic Goes After Axonics Again
Medtronic has made fresh new patent infringement accusations against Axonics.
Sign up for the QMED & MD+DI Daily newsletter.