Morari Medical is developing a wearable device that could make premature ejaculation a thing of the past. The company debuted a prototype of the technology during the Consumer Electronics Show earlier this month.
January 17, 2020
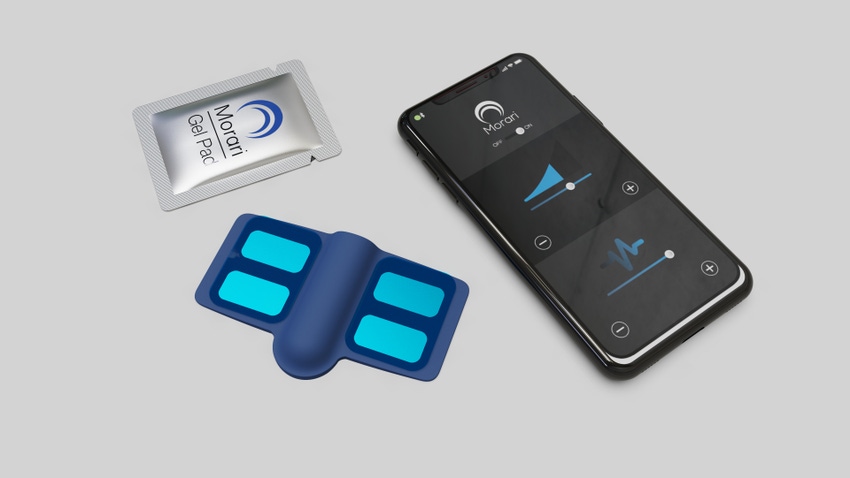
Men’s sexual health and wellness company, Morari Medical is using neuromodulation for its wearable device to help treat premature ejaculation (PE).
The Maple Grove, MN-based company debuted a prototype of the patch-like device at the Consumer Electronics Show in Las Vegas earlier this month. Morari was one of 10 companies in the emerging sextech market to have a presence at the show.
Jeff Bennett, the company’s founder and CEO spoke with MD+DI about the Morari Medical and its mission.
“Specifically, we’re trying to address the medical condition of PE, which affects 30% of men,” Bennett told MD+DI. “With my prior experience with working at Medtronic and having experience with neuromodulation products, I knew that the use of electrical energy can use or affect many different clinical disorders including the brain for Parkinson’s; the bladder for overactive bladder; and the heart for arrhythmias. So, I thought why not take it from there.”
The disposable device is placed on the perineum - “the area between the anus and the posterior part of the external genitalia.”
Here’s how it works.
“We’ve got a flexible circuit board that can be flexed to fit in the perineum area and on that board are electrodes that receive electrical energy from a battery that’s within the device. This battery will send energy to the electrodes and also send energy to the nerves in that area and cause that normal nerve function to temporarily be confused or altered. When the device is turned off it resumes normal nerve activity. We’re not curing for PE. This is on-demand.”
Bennett added, “this device is put on prior to penetration. It’s similar to when you would put a condom and then it’s worn during intercourse. After ejaculation has occurred it’s removed and disposed of. The feeling of electrical impulses isn’t painful.”
He said there are two paths the technology could take in getting to the market.
“We hope we can use the guidance that FDA has provided as a general wellness product and go to market under that classification, which wouldn’t require any approval but would limit the claims we would make,” Bennett said. “We couldn’t say premature ejaculation, but we could talk about improved sexual performance; and improv[ing] a partner’s satisfaction. Our current intent would be to go to market under the general wellness category and then follow that up with a study we could go to with FDA and get a specific indication and makes some claims around PE.”
He added, “the feasibility study will be the first study we’ve done to measure any sort of efficacy and change. But we fully anticipate that we’ll get positive results and then we’ll move into working into commercialize the design and build up the scale to then move into a wellness launch. We anticipate the wellness launch could be in mid-to-late 2021. In parallel, we would be doing the studies to get the data that we could go to FDA and ask for a specific indication for [PE].”
Sex and Medtech
There are a number of medical devices on the market that have been used to improve sexual function in both men and women.
In July of last year, Boston Scientific said it had developed Tactra, a malleable penile prosthesis used to treat erectile dysfunction. Marlborough, MA-based Boston Scientific said it is the first new innovation in penile implants it has developed in more than a decade. FDA cleared the device in April.
Nearly two years ago, Viveve Medical won a nod from FDA to continue enrollment in a clinical trial to evaluate a technology to improve sexual function in women following vaginal childbirth. The Englewood, CO-based company has an RF-based technology to treat vaginal laxity that could occur following childbirth.
About the Author(s)
You May Also Like




