ASTM has published F3502-21, a standard specification for general-purpose face coverings, which could help consumers understand covering filtration efficiency and more.
March 4, 2021

When the pandemic started, there were many questions about how to protect ourselves and others. Face masks have been cited in many cases as a first line of defense for those who cannot stay home, and mandates requiring face coverings have been issued in many locations throughout the world. With FDA-approved face masks sometimes in short supply, the general public has turned to various other sources for face coverings. People can be seen using everything from homemade masks, to neck gaiters, to masks made by their favorite clothing companies. As the pandemic evolved and we learned more about the disease and its spread, recommendations changed slightly. The effectiveness of some of these improvised face coverings was questioned, and the need for a standard to help the public determine the effectiveness of these products and to set a minimum requirement for some physical properties became more obvious.
On February 17, 2021, ASTM published ASTM F3502-21, a barrier face covering standard for general-use masks. This is a huge step forward in ensuring that masks used as barriers for the public meet a minimum performance requirement. The standard is separate from either the NIOSH respirator standards for N95, N99, and N100 respirators or the ASTM standard requirements for medical face masks. While N95 respirators offer the most protection, it is not practical for the general public to wear them owing to supply-chain concerns. FDA-cleared face masks are important equipment for medical use, and while they might be appropriate for the public as well, the FDA approval process is an expensive and time-consuming one. Using medical devices in daily life could result in a shortage in healthcare facilities where these products are needed most. This new standard fills an important gap made obvious by the pandemic. In times when the general public may be asked to don face coverings for the protection of those around them, this standard will give consumers the peace of mind they need, knowing that the masks they are using have been evaluated for safety and have passed minimum requirements. Table 1 below gives a quick explanation of each mask type and its primary intent.
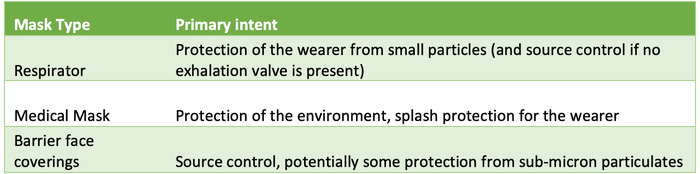
Table 1. Mask type and primary intent.
Testing requirements to certify a respirator with NIOSH or to market a medical face mask with FDA clearance have long been in place. Testing for respirators requires sub-micron filtration efficiency testing using particles with a mass median aerodynamic diameter of 0.3 microns. This is the size expected to be the most-penetrating particle size for this type of testing and is used to ensure a worst-case expectation during testing. Testing takes place at a flow rate of 85 liters per minute (L/min) and evaluates the filtration efficiency of the respirator. Inhalation and exhalation resistance are measured for respirators, and if exhalation valves are present, they must be tested for leakage. A fit test is required for all NIOSH-approved respirators to ensure the product seals well to the face. If the product does not fit the face snugly, the product is not as effective owing to leakage. Respirators are periodically tested to ensure continuing quality and performance. Particulate respirators are divided into three categories (N95, N99, N100) according to their performance on the filtration efficiency test.
Medical face masks cleared by FDA are subjected to a separate set of test requirements. Sub-micron particulate filtration efficiency testing using a different method is required for clearance along with bacterial filtration efficiency testing, differential pressure (breathability) testing, flammability testing, and synthetic blood penetration testing. Different combinations of results are used to separate these products into three levels, each of which is appropriate for different applications in medical use.
The ASTM F3502 Standard Specification for Barrier Face Coverings makes it clear that these products are neither medical masks nor respirators but are in another category altogether. This specification includes a description of what types of products should be evaluated using this process and how testing combined with smart design will result in a product that is best suited for these purposes. Products that should be evaluated using this standard are for source control (reducing droplets and particles expelled by the wearer) and may also provide some protection for the wearer from the inhalation of small particles. These products should not be used in medical settings and are not considered respirators. Their design should cover the nose and mouth of the wearer and omit any exhalation valves or any other design integration that would allow air to bypass the filtration mechanism. Design should also address fit and ensure that the product fits snugly to minimize leakage. Leakage will be assessed by this design analysis but may also include a quantitative analysis of leakage according to ASTM test method F3407. Additionally, the standard specifies the necessity of having a way to secure the covering to the user’s face, and sizing to aid in the appropriate fit of the product. Instructions for choosing the appropriate size should also be included. For multiple-use products, instructions for laundering and the maximum number of laundering cycles will also be included.
The required testing for barrier face coverings is limited to sub-micron particulate filtration efficiency and inhalation air flow resistance. Filtration efficiency testing is performed using the same equipment and basic principles as are used for testing NIOSH approved respirators. The two performance levels specified in Table 2 indicate the minimum filtration efficiency requirements to meet each level. As you would expect, a higher filtration efficiency would indicate that fewer particles pass through the product. Air flow resistance testing measures the air permeability of the product and is more of a comfort factor than a performance factor. A lower value means a more breathable (more comfortable) product. Requirements for the two performance levels are indicated in Table 2.

Table 2. Performance requirements according to the proposed barrier face covering standard.
Products may comply with either level for each test result and should be labelled accordingly. This means there may be products that perform at Level 2 for the sub-micron particulate efficiency test, but only Level 1 for the air flow resistance test. Performance should be indicated according to the results of each test.
While only two performance tests are required by the standard, optional testing is included as well. Inward leakage may be assessed solely through design, but a quantitative inward leakage test method is also available. Bacterial filtration efficiency may also be assessed. This testing uses a live bacterial culture suspended in particles of approximately 3 microns for testing. These tests may give more information about the performance of a barrier face covering but are not required to claim compliance with the standard.
While compliance to this standard is voluntary, it is important to have a tool for ensuring a way for the public to determine which products are best for them. Having a standard that sets minimum criteria and gives two levels of potential protection is a boon to anyone who has wondered about the effectiveness of their mask.
About the Author(s)
You May Also Like




