October 9, 2013
Most medical device firms pay lip service to process improvement initiatives, research from Axendia, an analyst and strategic advisory firm. But they often are stuck in a "firefighting" mode that leads them to chase after defects. "Reducing defects should be an outcome of a well designed and executed process, not the drivers for them," said Daniel Matlis, president of Axendia Inc. in a recent Qmed webinar detailing recent research carried out by the firm. The findings were in line with 2012 research carried out by the firm regarding medtech globalization that found that 59% of medical device professionals "worry about maintaining consistent quality standards across internal and external sites."
The 2013 study, which involved 239 medical device professionals based in 22 countries, reports that 88% of those polled found that defects were a form of waste with a medium or high impact on the organization. This strategy fails to confront the root causes of defects, causing similar problems to reappear. One of the key challenges in addressing this issue was a lack of resources, which was a problem cited by 74% of participants.
While the majority of medtech firms have process improvement methodologies in place, these initiatives often don't receive the attention they deserve. Again, the cause of this is that the bulk of medical device firms use a "brute force" strategy to confront product development problems and achieve regulatory compliance.
Axendia recommends that medical device companies commit to long-term process improvement efforts that receive the backing of the entire organization and outsourcing partners. Such a focus has been rare for public companies, which focus acutely on next-quarter earning results and lose long-term focus. A more effective requires a broader view and interconnection across the enterprise that enables device firms to detect problems early before they morph into non-conformances, CAPAs, recalls, or other problems.
|
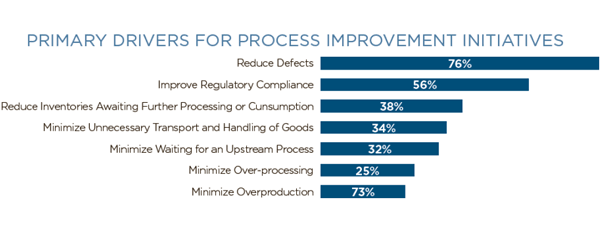
Of those polled in the Axendia study, 76% reported that reducing defects was the main reason to implement process improvement initiatives. |
Axendia recommends that device companies ensure that IT systems support process improvement by integrating data islands and point systems into a manufacturing-execution-systems-like system to organize data to support strategic operations and business decisions. Following such a strategy will enable the company to shift from a reactive to a proactive and ultimately predictive strategy in confronting product defects and other problems.
On a related note, Dale Wahlstrom, president and CEO of Lifescience Alley will take up a related topic at the upcoming BIOMEDevice San Jose conference in December. In a talk titled "Regulatory Science as a Platform for Faster and Cheaper Market Entry," Wahlstrom will discuss how device firms can increase efficiency and become more innovative by improving their product development efforts.
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
Related Content
About the Author(s)
You May Also Like