October 10, 2013
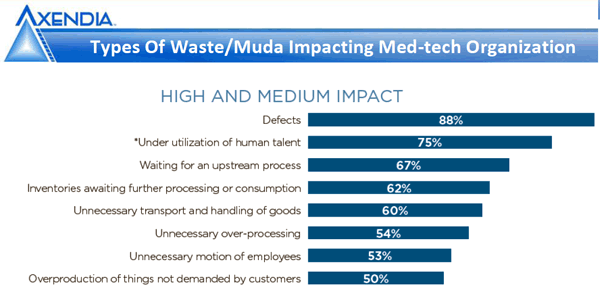
Defects are rising sharply in the medical device industry as a recent Axendia study indicates. In addition, medical device recalls recently hit a five-quarter high. A few main reasons contributing to these trends are increasing device complexity, software errors, and the complexity of outsourcing production. In consumer electronics, these issues are ongoing occurrences. The CE industry must constantly innovate and break new ground to keep retail placement (6-month resets) and consumer relevance. There were many policies and procedures I've used that were above and beyond industry standards to ensure the we and our customers were receiving the level of quality we expected. ISO does not guaranty quality or performance and its safe to say we found deficiencies even when working with Tier 1 global manufacturers producing millions of units for us.
 The automotive industry hit a similar technology wall approximately 15 years ago when micro-controllers started being used. Aggressive regulations on fuel economy, emissions, and performance tracking pushed more rapid adoption of new technologies. As the OEMs developed or found solutions to these problems they were able to reduce development cycles and regulatory approval times, improved operating efficiency and resource utilization and most importantly product quality was raised to competitive levels (especially when compared to Japanese benchmark).
The automotive industry hit a similar technology wall approximately 15 years ago when micro-controllers started being used. Aggressive regulations on fuel economy, emissions, and performance tracking pushed more rapid adoption of new technologies. As the OEMs developed or found solutions to these problems they were able to reduce development cycles and regulatory approval times, improved operating efficiency and resource utilization and most importantly product quality was raised to competitive levels (especially when compared to Japanese benchmark).
In my opinion, the underlying issue is data awareness. Today, autos have approximately 100 electronic control modules running 100 million lines of code that are connected to hundreds of sensors that generate up to 25 GB of data per hour of operation. This necessitates a tremendous amount of data testing and monitoring capability, which has grown exponentially over the last 15 years. Data is analyzed that is generated from initial R&D, through manufacture and continues throughout the entire product life cycle.
On the other hand, we've heard medtech personnel say they were "data poor." Medtech can learn from processes used in consumer electronics to ensure that quality meets expectations (especially from multiple outsourced vendors) and employ some of the technologies created to validate software performance, reduce the burden of software and continually test performance from cradle to grave. With outsourced vendors they are at the mercy of many different vendors and their typically proprietary QMS. It is nearly impossible to integrate all available data sets and truly understand the quality potential of a product, let alone identify the presence of a problem quickly and resolve it in a timely manner.
|
A recent report from Axendia (summarized in this Qmed link) finds that defects make up the most significant type of medium- and high-impact waste in the medical device industry. |
In the era of Big Data we often hear that there is a goal of understanding 1% of the available data and that only 25% has any potential value. Medtech needs the tools that enable them to control their device generated data across the entire global ecosystem to ensure quality and compliance and inevitably patient safety. The good news is they exist and they are able to learn from the development path in other industries which should offer substantial and rapid improvements throughout their organization. In the current market climate, I think this could make a significant difference in their ability to grow and prosper while gaining customer (user facilities and patient) confidence.
Bill Acevedo is the CEO of MedLogiq. He has more than 25 years' experience in the consumer electronics industry where he has worked in business development, program management, operations, quality control, and support systems.
About the Author(s)
You May Also Like