June 1, 2017
Zimmer Biomet recalled two implantable spinal fusion stimulators after a routine cytotoxicity test indicated high levels of chemicals that could be toxic to tissues or organs.
Amanda Pedersen
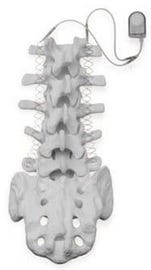
Zimmer Biomet is recalling its SpF PLUS-Mini and SpF XL llb implantable spinal fusion stimulators due to higher than allowed levels of potential harmful chemicals, which may be toxic to tissues or organs. The company initiated the recall on April 20 after discovering the problem during routine monitoring, and FDA identified it this week as a Class I recall, which is the agency's most serious type of recall.
|
Zimmer Biomet recalled its SpF PLUS-Mini and SpF XL IIb implantable spinal fusion stimulators after routine testing revealed harmful chemicals at levels that could be dangerous to the patient. |
The devices being recalled are used during spinal fusion surgery to increase the possibility of permanently connecting two or more backbones together. The device is implanted into the patient's back and is designed to provide constant electrical stimulation to the surgical site.
Companies use a cytotoxicity test as part of the biological evaluation of medical devices to ensure compatibility with the device and the human body. A failed test can indicate that a device contains potential harmful chemicals at amounts or levels that could be dangerous to the patient. Using the product could cause serious adverse health consequences such as chronic infections, long-term hospitalization due to additional surgeries, paralysis, and death, FDA noted.
As part of the recall, Zimmer reminded surgeons that normal clinical monitoring is recommended for three to six months after surgery for any patient with the affected devices implanted.
This is Zimmer's second recall this year that FDA has identified as a Class I recall, the first being a shoulder replacement device. In total, FDA has reported 20 medical device recalls in 2017, which is consistent with the rate of device recalls last year. As of June 1, 2016, there had also been 20 medical device recalls identified by FDA.
Besides Zimmer, companies that have had more than one recall this year, according to FDA, are Medtronic with four recalls, Abbott with two recalls, and Physio-Control with two recalls.
Amanda Pedersen is Qmed's news editor. Contact her at [email protected].
[Image credit: FDA]
About the Author(s)
You May Also Like