Visura Technologies has received FDA clearance for a disposable camera that can help physicians view the upper airway during transesophageal echocardiogram (TEE) procedures.
July 11, 2018
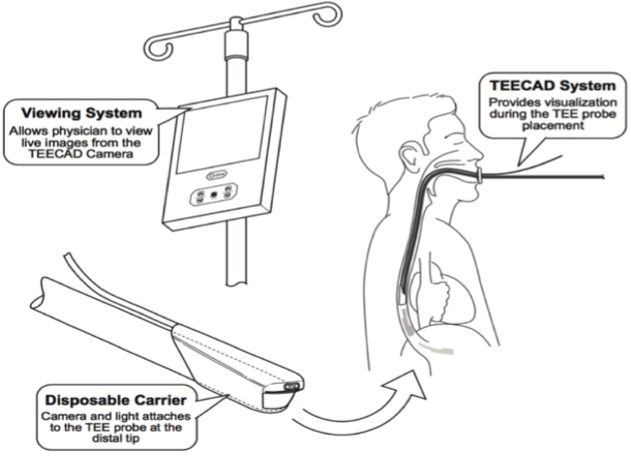
A start-up company is hoping to take the guess work out of transesophageal echocardiogram (TEE) procedures. Visura Technologies said it has developed TEE Camera Assist Device (TEECAD) System, a single use disposable camera that easily attaches to a TEE ultrasound probe allowing physicians to view the upper airway and esophagus during probe placement for safe intubation.
The Evanston, IL-based company recently received FDA clearance for the device. David Marmor MD, the founder and CMO of the Visura Technologies said prior to the clearance physicians had were operating blind during TEE procedures.
“We would place the probes down the esophagus by physician feel and patient cooperation – we weren’t able to see where we were going,” Marmor, told MD+DI. “There are certainly tricks you can use to make it a little easier, but at the end of the day you’re still doing a blind procedure.”
Marmor added that theTEECAD system is going to take the guess work out of the procedure. "We hope it’s going to have a higher rate of success for first attempt intubation of the esophagus,” he said.
The TEECAD System consists of a single-use disposable camera Carrier that removably attaches to the TEE probe, and a separate Viewing System display that allows the physician to view real-time images from the camera to visually assist with safe probe intubation.
The clearance of Visura’s first TEECAD camera Carrier is for use with the Philips X7-2t probe. The company said it plans to develop additional Carrier models compatible with other TEE probes available in the market.
Visura was founded in 2015 and had been in development mode for a few years now.
“We submitted our 510(k) filing in April, so getting the [clearance] recently is a huge milestone for the company,” Eric Sandburg, Visura Technologies CEO, told MD+DI. “The 510(k) is our first regulatory [clearance] and we do plan to pursue CE mark in 2019.”
About the Author(s)
You May Also Like




