Real-time visualization of hemoglobin oxygen saturation (StO2) levels in tissue could help surgeons better identify potential ischemia.
July 22, 2021
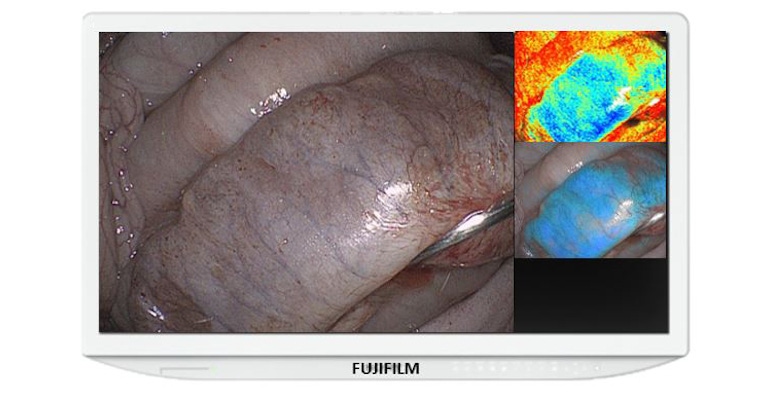
Fujifilm has just earned 510(k) clearance for a new enhancement to its ELUXEO Surgical System that provides real-time visualization of hemoglobin oxygen saturation (StO2) levels in tissue using laparoscopic and/or endoscopic imaging to help surgeons quickly detect ischemic tissue. The system had previously received an FDA Breakthrough Devices Designation late last year.
Surgeons have traditionally relied on fluorescent imaging using indocyanine green (ICG) dye to understand blood flow, Stephen Mariano, Global VP, Endosurgical R&D, Fujifilm Medical Systems, USA, told MD+DI. “The fluorescent imaging using ICG is a method in which an injectable dye circulates through the blood vessels, helping to identify tissue perfusion, but there are limitations to this technique: ICG needs to be intravenously injected into a patient prior to the imaging, [and] surgeons face time constraints during fluorescent observation due to filtration of ICG dye in the liver.”
The current ICG fluorescent imaging systems also do not allow physicians to visualize blood flow during endoluminal procedures, Mariano said. “This is an issue as the industry is trending towards performing more procedures endoscopically, and as minimally invasively as possible,” he said.
The Endosurgical Image Enhancement Technology combines advanced light engines along with image and video processing power to enable real-time visualization of tissue oxygenation, Mariano said. “The benefit of this technology goes beyond the imaging and visualization afforded by the light source,” he said.
The technology will be available shortly to surgeons as an upgrade to the ELUXEO Surgical System. The full suite of ELUXEO image enhancement tools will include Blue Light Imaging (BLI) and Linked Color Imaging (LCI) modes as well as the new StO2 visualization mode. Each image enhancement technology provides physicians with unique data allowing them to better diagnose issues during procedures, FujiFilm reported in a news release.
Real-time visualization of StO2 levels could prove particularly useful for gastrointestinal procedures. “Being able to visualize StO2 levels is critical for surgeons and endoscopists, especially those who perform procedures involving (but not limited to) the gastrointestinal tract, where the potential for tissue ischemia is of high concern,” Mariano said. “Tissue ischemia can lead to tissue necrosis if undetected or untreated, and necrotized tissue will allow breakdown of the anastomosis or weaken the bowel wall and may lead to perforation. Leakage of GI contents can cause systemic sepsis and multi-organ failure, and may lead to death in serious cases.
“Fujifilm believes this technology will provide surgeons with another data point when managing GI anastomoses. This visualization also provides an added level of confidence to help minimize readmission rates as a result of GI anastomotic leaks,” he added.

Such visualization may also help drive the growing shift towards minimally invasive surgery (MIS), in terms of both laparoscopic and endoluminal procedural approaches, said Mariano. “More and more surgeons are including endoluminal procedures in their MIS practices to deliver quality, but less invasive care to their patients. This technology enables visualization of oxygen saturation during endoluminal procedures which otherwise would not be possible,” he said. “In addition, the Fujifilm system intends to further MIS procedures by providing a greater degree of confidence to surgeons when performing certain complex MIS procedures.”
Receiving the Breakthrough Device Designation from FDA contributed to an accelerated assessment and interactive review of Fujifilm’s technology, said Mariano. “The goal of the Breakthrough Devices Program is to provide patients and health care providers with timely access to these medical devices by speeding up their development, assessment, and review, while preserving the statutory standards for premarket approval, 510(k) clearance, and De Novo marketing authorization, consistent with the Agency's mission to protect and promote public health. We appreciate the validation and confidence this designation instills in our technology.”
About the Author(s)
You May Also Like




