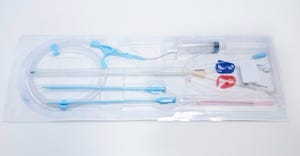
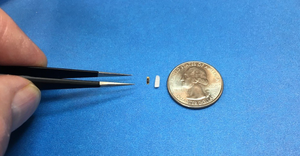
Contract Manufacturing
Nitinol medical device use
Manufacturing
Addressing the Rising Price & Demand of NitinolAddressing the Rising Price & Demand of Nitinol
Novel applications and a resurgence of minimally invasive procedures are causing increases in the expense of this elastic alloy. But industry tactics are aiming to curb the costs.
Sign up for the QMED & MD+DI Daily newsletter.

.png?width=700&auto=webp&quality=80&disable=upscale)
.png?width=700&auto=webp&quality=80&disable=upscale)
















![Featured Image] 230916SF73.jpg Featured Image] 230916SF73.jpg](https://eu-images.contentstack.com/v3/assets/blt14ac89070d5e4751/blt381af30a9066bd0b/655b99d3323a8f040a9ee156/Featured_Image_230916SF73.jpg?width=300&auto=webp&quality=80&disable=upscale)