Equipment
thumbnail
Equipment
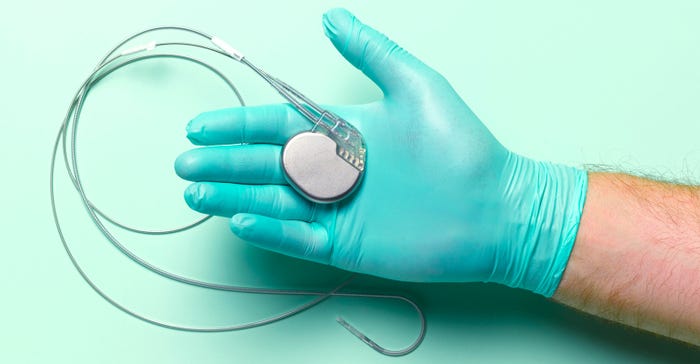
Getting to the Heart of What the Medical Battery Market NeedsGetting to the Heart of What the Medical Battery Market Needs
Wyon's Marcel Inauen discusses the challenges of miniaturizing power sources and how to overcome the hurdles.
Sign up for the QMED & MD+DI Daily newsletter.