|
|
|
Karl J. Hemmerich is the manager of plant operations for STERIS Isomedix (Sandy, UT), and John Masefield is executive advisor to STERIS. Jerry R. Nelson, PhD, is director of Nelson Laboratories Inc. (Salt Lake City). |
Over the past 25 years, the medical device industry has changed significantly. Sterilization has evolved as well, sometimes reacting to the industry and sometimes leading the way in new methods, standards, and processes. In the eyes of someone looking forward in time from 1979, many medical devices today are very sophisticated in nature and design while others hardly changed at all. Sterilization technologies, having remained unchanged in the physics and chemistry of microbial inactivation, have undergone a different sort of metamorphosis than the medical products they support.
In 1980, the primary methods for medical device sterilization were ethylene oxide (EtO), gamma, electron beam (E-beam), moist heat, dry heat, liquid-chemical germicide, and other gaseous methods. More than 20 years later, the primary sterilization modalities remain the same. So, has nothing changed?
Each sterilization method kills organisms effectively, and usually one method is more effective or more efficient for a given product than another. However, changes in the methods themselves, the standards, and the development of biological indicators (BIs) and parametric release have led to modifications in the processes and shifts in industry preferences. This article explores those changes and their effects over the past 25 years on the medical device sterilization market.
Sterilization of Medical Devices
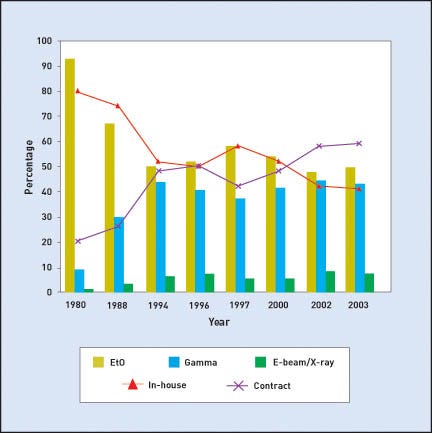
In 1980, EtO was used for nearly 90% of all sterilized devices. At that time, only the largest manufacturers used either gamma or E-beam sterilization. Irradiation contractors were just emerging with a limited number of facilities. In fact, the two largest contract irradiators, STERIS Isomedix and Sterigenics, were only founded in the 1970s. Figure 1 shows the usage breakdown for EtO, gamma, and E-beam, as well as for in-house and contract sterilization, from 1980 to 2003.
One aspect of the medical device industry that is hidden in the data is that an ever-increasing portion of U.S. and Canadian medical device manufacturing is moving overseas. However, many of those foreign-produced products return to North America for terminal sterilization.
The reason for this pattern relates to both the corporate structures and the difference in the capital investment required for manufacturing operations compared with sterilization. Manufacturing often requires minimal costs for both labor and capital equipment, whereas sterilization facilities require substantial capital for equipment.
The Role of Standards. The development of standards and guidelines has been critical to advances in sterilization. Both the Association for the Advancement of Medical Instrumentation (AAMI) and the International Organization for Standardization (ISO) have developed viable validation guidelines that are relatively easy to execute.
These standards often came in response to FDA and international regulatory requirements related to good manufacturing practices and quality assurance. The Medical Device Amendments of 1976 were followed by FDA's “Reproduction Quality Assurance Planning” guidance documents in 1981 and 1988 and its “Guideline on General Principles of Process Validation” in 1983 and 1987, which changed the way sterility was addressed and validated. These were augmented by the 1990 amendments.
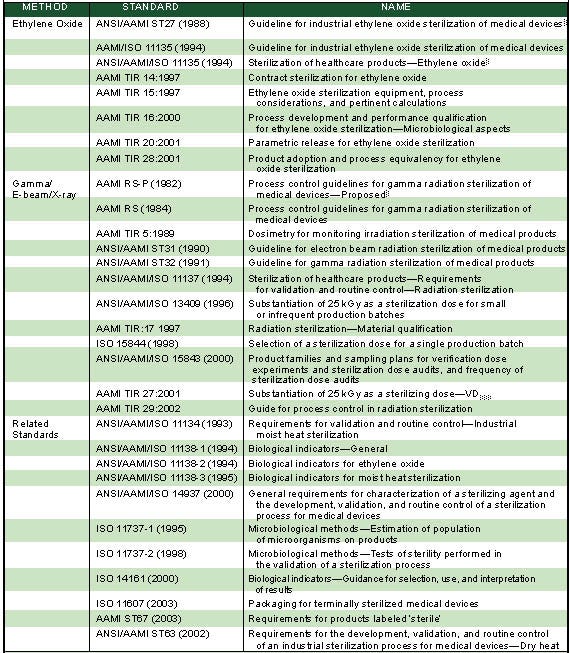
Today, the AAMI and ISO guidelines enable companies to implement a sterilization program without the need for a large and burdensome sterilization technologies department. These voluntary consensus standards have also provided regulatory bodies such as FDA a reference on which to base their determination of product sterility. Table I provides a listing of current AAMI and ISO sterilization standards.
Contract Sterilization. In response to market demands, contract sterilization services have increased significantly. The growth of sterilization contract services has been a result of the drive for medical device manufacturers to decrease costs.
The availability of contract services has played a major role in the development of sophisticated medical device products. Without having to invest in the equipment or labor required, companies have access to the state-of-the-art sterilization facilities that are necessary for such products.
The convergence of these events fueled the progress of sterilization over the past 25 years. Improving turnaround time is often a requirement for survival. Methods, systems, and guidelines have been developed to fulfill the just-in-time manufacturing methods in widespread use today. Parametric release and instantaneous BIs are just two of the breakthrough concepts being developed to reduce turnaround time and improve the reliability of sterilization for manufacturers.
EtO Sterilization
EtO sterilization has been critical to the sterilization of disposable medical devices since its discovery by Phillips and Kaye in 1944. They stumbled upon it while conducting work on biological decontamination at Fort Detrick, MD, for the U.S. Army. In the 1980s, EtO lost some market share to irradiation sterilization as a result of environmental laws (e.g., Montréal Protocol [1987], California Prop. 65, California Air Quality Management District, and other EPA regulations) that were designed to curtail the destruction of the earth's ozone layer by fluorinated gases.
At the time, many thought that EtO would be phased out; however, the decreased use proved to be just a temporary setback as companies implemented the required changes to switch from 12/88-EtO/chlorofluorocarbon-gas mixture to 100% EtO gas. Since then, EtO sterilization has grown steadily as it benefited from the ANSI/AAMI/ISO Guidelines ST27/11135-1994, existing capital investments, minimal retrofit costs, and contractor availability.
Major advances in EtO sterilization include improved BIs, parametric release, lower gas concentrations, and improved outgassing methods. BIs have evolved from earth- or sand-borne bacteria in EtO's early years to protein-based alternatives with ever-faster response times. In fact, the parametric release process has decreased from 10–14 days, which was common for BI release in the late 1970s, to the current 2 days. Even the dread of dealing with EtO residuals has abated through the use of nitrogen flushes, cycled heat washes, and other processing methods.
Parametric release, which was implemented in a few in-house 12/88 EtO sterilization facilities 20 years ago, is now in place in many in-house and contract facilities. The advent of intrinsically safe infrared spectrophotometers, as well as computer-controlled systems, has enabled parametric release in 100% EtO processes. Enhanced BI incubation time reduction studies, lower residuals, and parametric release have ultimately led to safer products and shorter product holding times.
Gamma Sterilization
|
Table I. An overview of global sterilization and related standards (click to enlarge). |
Gamma sterilization was a post-WWII technology. During the early 1960s, it was used initially to eradicate anthrax on wool for carpet
manufacturing in Australia. Gamma was not used to sterilize medical products in North America, however, until 1964. The Atomic Energy of Canada (later MDS Nordion; Ottawa, ON) commissioned the first industrial cobalt-60 sterilization facility to sterilize surgical sutures for the Ethicon division of Johnson & Johnson.
In 1972, Isomedix (later STERIS Isomedix) opened its first contract irradiation facility in New Jersey. Sterigenics opened its first gamma facility in 1979. A few large medical device manufacturers invested in this new technology around the same time.
The development of radiation-stable and enhanced-performance polymers was key to the success of gamma sterilization. Today, PVC and polycarbonate formulations eliminate the brown-yellow color that developed with predecessor products.
Radiation-stable polypropylene has been developed to prevent the brittleness that previously limited the polymer's use in products that would be radiation sterilized. AAMI Technical Information Report 17 was published in 1997 to define materials stability, processing effects, and proper test qualification methods (including accelerated aging).
Validation methods have also improved. Guidelines have progressed from “just use 2.5 Mrd” to the Kilmer method, to ANSI/AAMI ST32 (1980/ 1990), to AAMI/ISO 11137 (1994), and AAMI/ISO 13409 (1996), and finally, to the current TIR 27 VDmax. In 1974, under the auspices of AAMI, a committee was formed to develop rational process-control guidelines for the sterilization of medical devices.
The validation guidelines that were developed included the development of rational dose-setting methods that took into account actual product bioburden, its distribution on the product, its radiation resistance, and, to a limited extent, the end-use of the product. By 1994, after much work and a series of national and international meetings, the AAMI dose-setting methods received worldwide recognition when included, with little modification, in ISO 11137.
Use of these rational dose-setting methods frequently resulted in sterilizing doses much lower than the traditional 2.5 Mrd, which further expanded the range of conventional medical-grade plastics that could be sterilized without generating unacceptable color, hardness, or brittleness. It is important to note that the unit for irradiation dosage has changed from megarads (Mrd) to kiloGrays (kGy) in order to comply with System International conventions.
By the mid-1990s, a growing number of contract gamma irradiation facilities were making sterilization services available to medical product manufacturers, making radiation sterilization more cost-competitive.
A national network of large-scale contract irradiation facilities has meant that healthcare companies no longer need to build in-house irradiators. Many device companies have decommissioned their in-house irradiation facilities in favor of using contract irradiation facilities.
Mathematical Modeling. Manufacturers are increasingly interested in the mathematical modeling of dose distribution in irradiated products. Such modeling may help improve process design, evaluation, and control. Manufacturers hope to better understand the distribution of the absorbed dose within products of heterogeneous density when the products are exposed to ionizing radiation in an industrial irradiator. They also hope to minimize the number of physical dosimeters required.
The use of dosimetry to measure the actual dose applied during processing has also evolved. The use of extensive numbers of thin films and dyed-plastic dosimeters has shifted to the less-frequent use of more-accurate reference alanine dosimeters in production irradiation runs. Predictive mathematical models have been developed for gamma, high-energy E-beam, and x-ray irradiation facilities. As determined by the mathematical modeling, the dosimeters are placed in zones of minimum and maximum dose in the products being irradiated. Other dosimetry developments include Internet-based dosimeter calibration and real-time monitoring of absorbed dose.
E-Beam and X-Ray Sterilization
|
Figure 1. The bars for each year show the market share for EtO, gamma, and E-beam/ |
The biggest single change in the E-beam market has been the improved reliability of the delivery systems. IBA Inc. revolutionized the E-beam market with its Rhodotron accelerator, while Titan Scan Systems improved the reliability of linear accelerator design. Besides the accelerators themselves, materials-handling systems made a quantum leap to better present the product to the beam consistently and accurately.
X-ray has only recently been used for commercial sterilization. However, it was actually the first radiation technology for sterilizing medical products in the late 1800s. E-beam found its first medical niche in 1956 as a sterilization method when Johnson & Johnson used it for sutures.
Recent developments have started looking at x-ray for use with 5–10 million electron-volt (MeV) high-power electron accelerators, with the goal of enabling the sterilization of higher-density medical products and the terminal sterilization of heat-sensitive, low-density medical products.
Progress is being made in the development of modular, sealed E-beam tubes, working at voltages around 100 kV. The tubes show promise for low-power use, particularly in the surface sterilization of medical devices and pharmaceutical packaging materials used in conjunction with aseptic filling operations.
Vaporized Hydrogen Peroxide
Vaporized hydrogen peroxide (VHP) sterilization is increasingly being used for the industrial sterilization of medical devices. As a low-temperature gaseous method of sterilization, it is an alternative to EtO for some products. It does not penetrate as much as ethylene oxide does; however, it does offer some advantages, including excellent material compatibility and short cycle times. And, because peroxide breaks down into water and oxygen, there is virtually no aeration time. VHP is also sporicidal at very low concentrations (typically 1–2 mg/L at 25°C).
Like all sterilization modalities, VHP has limitations. Because it lacks the penetration available with EtO, an open gas pathway must be present, and certain materials (e.g., cellulose) cannot be processed.
Validation for VHP is similar to that for EtO. Because VHP sterilization has no consensus standard, the concepts that are outlined in ANSI/AAMI/ISO 14937 for general requirements of a sterilizing agent can be used to develop an appropriate validation strategy. Other published EtO standards can be used as well. Several companies have recently obtained 510(k) clearance to use VHP to terminally sterilize their medical devices.
Steam Sterilization
Medical devices manufactured from materials that are thermally tolerant can be processed with steam. The use of steam is increasing due to its inherent low processing and capital costs, direct in-house control, and parametric release; however, it remains a small percentage of the overall market.
Some sterilization contractors now provide steam sterilization services. In addition, a variety of packaging materials are available that can be
validated.
Dry-Heat Sterilization
AAMI recently released a new standard for validation of dry-heat sterilization. Dry heat has classically been considered as 170°–180°C for an hour; however, longer times at considerably lower temperatures also can be validated. An increasing number of medical devices are being processed with dry heat (160C), although its market share is not easily determined because most dry-heat processing is not outsourced. Packaging materials that can withstand the rigors of dry heat are also now available.
The Sterilization Future
Over the past 25 years, sterilization has evolved to keep pace with the medical device industry. Although sterilization technologies themselves have remained unchanged in their physics and chemistry, some significant developments have advanced the methods and processes. The future promises to offer even more substantial developments. Key developments in the sterilization market as a whole should include:
•Changes in the sterility assurance level (SAL) requirements for various device groups. Internationally, the requirement for a device to be labeled with a “sterile” statement requires a SAL of 10–6. In the future, this requirement may be revisited to allow products with alternate SALs (i.e., 10–3, 10–4, etc.), based on the need for such elevated SALs and on product use.
•Alternative gaseous-sterilization modalities, such as vapor-phase hydrogen peroxide and plasma vapor-phase hydrogen peroxide, offered on a contract basis.
•An overseas exodus of sterilization similar to that of device manufacturing.
•Modalities that are less energy dependent (i.e., more cost-effective) to combat spiraling energy costs.
•Improved manufacturing controls that reduce bioburden spike issues.
•Documents from the standards-writing groups covering how to deal with bioburden spikes.
•Rapid environmental monitoring techniques for manufacturing.
•Further harmonization of international methods.
Major advances affecting EtO sterilization and processing include:
•BIs that offer essentially instantaneous response times.
•Further developments in parametric release for EtO sterilization.
•Evolution of the industry guidelines for validation.
Developments in gamma sterilization will likely include:
•Final development and implementation of VDmax guidelines at doses other than 25 kGy with validation using this technique from 15 to 35 kGy.
•Evolution of the industry guidelines for validation. In 2004, the next revision of the AAMI 11137 standards should be released: AAMI 11137-1 draft standard, “Process Requirements for Radiation Sterilization;” AAMI 11137-2 draft standard, “Validation Methods for Radiation Sterilization;” and AAMI 11137-3 draft standard, “Dosimetry for Radiation Sterilization.”
•No sterilization dose validation requirement for devices with proven very low bioburdens.
•Predictive dosimetry using mathematical modeling that is based on the application of knowledge and computer power currently available.
•Parametric release for irradiation processing. Cost-competitive pressures and improved science (based on enhanced computer control and real-time dose-measurement systems) may drive this feasible methodology forward.
•Alternative dose-setting strategies, further increasing the options available to the sterilization microbiologist.
Laboratory advancements should include:
•Rapid microbiological methods that reduce the time needed for bioburden assessment from about a week to a day. The technologies already exist, but they will come into common use as regulatory acceptance, awareness, and their utility become better known.
•Genetic-based microbial identification methods as the standard for identification of environmental isolates, bioburden tracking, and failure investigations.
•Technology improvements such as isolators, cleanroom HEPA-filtered respirators and improved barrier gowns and gown materials that increase the reliability of existing sterility test methods.
•New rapid microbiological methods that replace the existing sterility test procedure.
With these developments, sterilization will continue to be an integral part of the medical device industry. As it has in the last 25 years, we are sure that MD&DI will bring you updates and developments as they happen in the next 25 years.
Copyright ©2004 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like