June 23, 2014
Adhesive gels
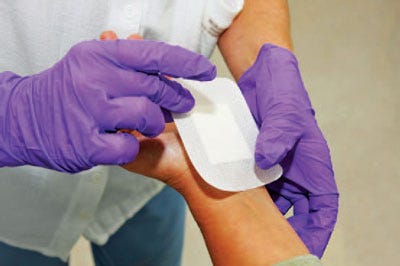 Medical-grade silicone sticky gels are customized for wound-management applications. Offered by Applied Silicone Corp., medical-grade silicone adhesive gels are optimized for scar-management products. Traditional methods for improving the aesthetics of hypertrophic and keloid scarring include cover-ups and laser or surgical procedures. Alternative cosmetic or textile covers can be messy and require an aggressive bond to the skin. In order to minimize the appearance of old and new scars, traditional silicone gel, in topical or sheeting form, offers the optimal combination of gentle, secure adhesion; a moisture barrier; and permeability. Adhesive gels are specially formulated silicone gels that possess similar properties of traditional silicone gels but with greater cohesive strength for durability. They can be cast or molded in unlimited thicknesses by virtue of a polyaddition cure mechanism that produces no by-products. Inherently hydrophobic, gels provide a strong water barrier, yet are permeable to water vapor and air. A breathable barrier surface applied over a scar will reduce its appearance by maintaining a clean, hydrated skin surface. Offering strong cohesion, adhesive gels are also resistant to tears and fractures in daily wear.
Medical-grade silicone sticky gels are customized for wound-management applications. Offered by Applied Silicone Corp., medical-grade silicone adhesive gels are optimized for scar-management products. Traditional methods for improving the aesthetics of hypertrophic and keloid scarring include cover-ups and laser or surgical procedures. Alternative cosmetic or textile covers can be messy and require an aggressive bond to the skin. In order to minimize the appearance of old and new scars, traditional silicone gel, in topical or sheeting form, offers the optimal combination of gentle, secure adhesion; a moisture barrier; and permeability. Adhesive gels are specially formulated silicone gels that possess similar properties of traditional silicone gels but with greater cohesive strength for durability. They can be cast or molded in unlimited thicknesses by virtue of a polyaddition cure mechanism that produces no by-products. Inherently hydrophobic, gels provide a strong water barrier, yet are permeable to water vapor and air. A breathable barrier surface applied over a scar will reduce its appearance by maintaining a clean, hydrated skin surface. Offering strong cohesion, adhesive gels are also resistant to tears and fractures in daily wear.
? Applied Silicone Corp.
SANTA PAULA, CA
www.appliedsilicone.com
Hydrocolloid dressings and bandages
 EuroMed Inc. offers a range of hydrocolloid dressings for the treatment of leg ulcers and pressure sores. It also provides a line of hydrocolloid bandages for the treatment of topical skin conditions. In addition, the company has introduced a line of skin-friendly ostomy elastic barrier strips designed to increase the security and wear time of ostomy appliances. The skin-friendly hydrocolloid adhesive, with its tapered edges and low-friction polyurethane film backing, is designed to enhance both wear time and security. The company can also develop custom wound-care products in an FDA-regulated Class 8 cleanroom.
EuroMed Inc. offers a range of hydrocolloid dressings for the treatment of leg ulcers and pressure sores. It also provides a line of hydrocolloid bandages for the treatment of topical skin conditions. In addition, the company has introduced a line of skin-friendly ostomy elastic barrier strips designed to increase the security and wear time of ostomy appliances. The skin-friendly hydrocolloid adhesive, with its tapered edges and low-friction polyurethane film backing, is designed to enhance both wear time and security. The company can also develop custom wound-care products in an FDA-regulated Class 8 cleanroom.
? EuroMed Inc.
ORANGEBURG, NY
www.euromedinc.com
Adhesives for wound-care applications
 Fabrico offers a range of adhesives for medical device applications, including acrylics, epoxies, and styrene block copolymers. The company's adhesives offer stretchability, conformability, breathability, absorbency, porosity, and durability. Featuring custom formulations, new classes of adhesives developed by the firm include hydrocolloid, hydrophilic, and conductive adhesives. These adhesives provide gentle adhesion to the skin, long-lasting bonds for extended wear, and--in some cases--the ability to withstand or absorb fluids. They also offer nontoxicity and adhesion to organic and inorganic surfaces. In addition, they are 100% solid before and after curing and are optimized for wetting and gap filling--characteristics that are suitable for high-volume production. These materials are compatibile with different forms of sterilization and offer antimicrobial properties. The company also offers hydrocolloids that are designed for wound-care applications requiring a precise moisture vapor transmission rate and extended wear.
Fabrico offers a range of adhesives for medical device applications, including acrylics, epoxies, and styrene block copolymers. The company's adhesives offer stretchability, conformability, breathability, absorbency, porosity, and durability. Featuring custom formulations, new classes of adhesives developed by the firm include hydrocolloid, hydrophilic, and conductive adhesives. These adhesives provide gentle adhesion to the skin, long-lasting bonds for extended wear, and--in some cases--the ability to withstand or absorb fluids. They also offer nontoxicity and adhesion to organic and inorganic surfaces. In addition, they are 100% solid before and after curing and are optimized for wetting and gap filling--characteristics that are suitable for high-volume production. These materials are compatibile with different forms of sterilization and offer antimicrobial properties. The company also offers hydrocolloids that are designed for wound-care applications requiring a precise moisture vapor transmission rate and extended wear.
? Fabrico
Kennesaw, GA
www.fabrico.com
Wound-care dressings
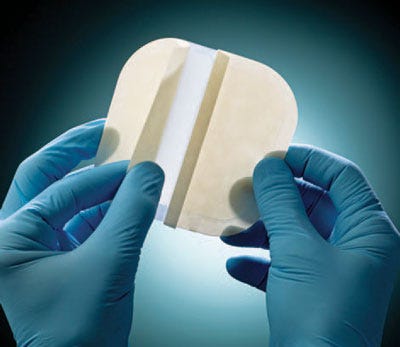 Vancive Medical Technologies has developed a wound-care portfolio that includes absorbent wound dressings. The BeneHold dressings are designed to manage lightly to moderately exuding chronic and acute wounds. The dressings maintain moisture within the wound and offer a self-adherent contact layer with a low-friction polyurethane top film to facilitate wound healing. The wound contact layer absorbs wound exudates and turns into a soft gel that protects against wound dehydration and facilitates granulation, epithelization, and autolysis. The top film is water resistant and acts as a barrier to external contaminants and bacteria. There are four types of wound dressings in the portfolio, which are offered in various sizes: a thin, absorbent wound dressing; a standard hydrocolloid dressing, a bordered hydrocolloid dressing with odor control, and a bordered sacral hydrocolloid dressing with odor control. In addition, the company has developed an adhesive containing chlorhexidine gluconate, which can be used for peripheral IV dressings. Unlike typical film dressings, this new dressing can absorb small quantities of exudate and remain transparent even when absorbing fluid. In vitro data have demonstrated a >4 log reduction on major microorganisms.
Vancive Medical Technologies has developed a wound-care portfolio that includes absorbent wound dressings. The BeneHold dressings are designed to manage lightly to moderately exuding chronic and acute wounds. The dressings maintain moisture within the wound and offer a self-adherent contact layer with a low-friction polyurethane top film to facilitate wound healing. The wound contact layer absorbs wound exudates and turns into a soft gel that protects against wound dehydration and facilitates granulation, epithelization, and autolysis. The top film is water resistant and acts as a barrier to external contaminants and bacteria. There are four types of wound dressings in the portfolio, which are offered in various sizes: a thin, absorbent wound dressing; a standard hydrocolloid dressing, a bordered hydrocolloid dressing with odor control, and a bordered sacral hydrocolloid dressing with odor control. In addition, the company has developed an adhesive containing chlorhexidine gluconate, which can be used for peripheral IV dressings. Unlike typical film dressings, this new dressing can absorb small quantities of exudate and remain transparent even when absorbing fluid. In vitro data have demonstrated a >4 log reduction on major microorganisms.
? Vancive Medical Technologies
Chicago
http://vancive.averydennison.com
About the Author(s)
You May Also Like


