These tips from two industry professionals could help you navigate roadblocks as you develop clinical software solutions around your medical device.
November 22, 2021

As healthcare providers continue to embrace value-based care models, there is an increasing need for medical device companies to offer clinical software solutions around their devices.
A recent MD+DI webinar, sponsored by S3 Connected Health, offered an opportunity to learn why medical device companies should create impactful clinical software around their medical devices, what solutions deliver value (clinical decision support, medication delivery management, and home-based treatment management), and how to be smart about implementation. In additoin to hearing from Piotr Sokolowski, chief medtech strategist at S3 Connected Health, webinar attendees also heard from Tom Alexander, vice president of marketing and business development at Nipro Medical Europe, about his real-world experience in building clinical software solutions around medical devices.
5 key areas of implementing clinical software solutions around medical devices
The first area to consider, according to Sokolowski, is the clinician experience with the clinical software solution. If the clinician experience is not taken into consideration, the solution will likely have poor adoption, he said.
"The bottom line is, make user experience your top priority," Sokolowski said. "Strive for great consumer-like user experience, rather than technician-like or engineer-like user experience."
Another important consideration is making sure the clinical software integrates with electronic health records. Without that integration, the solution will lack full patient information and there will be gaps in the patient's records.
"This one is largely out of our control, but fortunately regulators have stepped in to help, finally," he said.
The third area Sokolowski highlighted is data analysis and insights, and he noted that if the solution lacks data analysis it will not be able to drive clinical insights.
"If we want to generate insights from data we can't, unfortunately, rely on EHRs to provide this functionality because the system hasn't been built for that," Sokolowski said. "So we have to actually implement a separate software layer on top of the EHR that will handle this particular function. This will also facilitate data integration with other data systems, for example, data from remote patient monitoring devices."
The fourth area, regulatory and cybersecurity, is especially important in today's connected world. If proper attention isn't paid to cybersecurity and meeting regulatory requirements regarding cybersecurity, "in the best case, it will cause costly implementation, in the worst case it will cause safety and security risks for our patients," Sokolowski said.
Finally, Sokolowski said medical device companies developing clinical software around their products must pay attention to deployment and provisioning, otherwise they will face barriers to roll out and costly operations.
"The advice here is to seriously consider cloud-based deployment, or at least architect your system to be able to support the migration to the cloud," he said.
Common blockers that are encountered when building clinical software solutions around a medical device
In an audience poll question, we asked webinar attendees about the common blockers they have encountered as they have tried to implement clinical software solutions around their medical device. The results, shown below, were quite surprising.
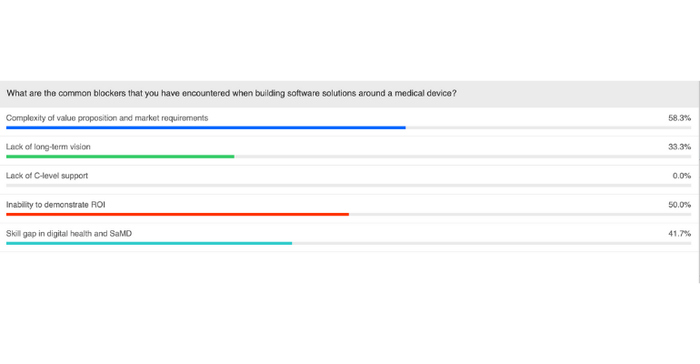
Perhaps the biggest surprise was that none of our poll respondents marked lack of C-level support as a blocker. As Alexander pointed out, however, most of the people dialing in to the live webinar probably have a predisposed interest in the topic and therefore have a better view on their organization's vision for implementing clinical software solutions.
"In general what I hear, at least [from] colleagues that are in the same situation trying to roll out these kinds of initiatives within their organizations is that the first item, the complexity of the value proposition, as well as the inability to demonstrate ROI usually have, as a consequence, the lack of C-level support," Alexander said. "So in the end, without ROI, without a value proposition, it's usually very difficult to get the C-level support to finance the initiatives."
He added that in his experiences with building clinical software solutions around medical devices, his team has encountered all of the above roadblocks. While none of them were major hurdles, they each needed to be tackled one by one, he said.
To hear more about how Alexander and his colleagues addressed each of these blockers on their journey, I encourage you to watch the on-demand version of this free webinar.
About the Author(s)
You May Also Like




