May 26, 2017
Roche's participation in the stock sale is seen as a good sign, with proceeds expected to carry the company through the FDA process by the end of the year.
Amanda Pedersen

Roche and other key investors are showing continued confidence in a Germantown, MD-based company that is trying to bring its implantable continuous glucose monitoring (CGM) system to the U.S. diabetes market. Senseonics said it raised about $41 million in a stock sale, thanks in large part to its international distribution partner Roche.
The company said the offering included about 29 million shares priced at $1.41 each, and that Roche has agreed to buy roughly 21.3 million of those shares. New Enterprise Associates also helped out by picking up about 7.1 million shares.
Senseonics said it will use the proceeds to launch its Eversense CGM in the United States, pending FDA approval, and to continue developing future configurations of the device. The company went public last year, and submitted a PMA application to FDA in October.
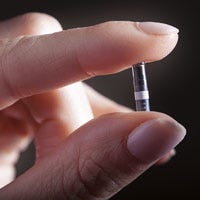
Senseonics landed a distribution agreement with Roche this time last year, giving the better-known diabetes player the right to sell the Eversense technology in Germany, Italy, and the Netherlands. The system consists of a long-term wear sensor, which is implanted subcutaneously and can be worn for up to 90 days, a transmitter, and a mobile app.
|
Senseonics' Eversense CGM consists of an implantable, long-term wear sensor (up to 90 days), a transmitter, and a mobile app. |
The offering comes at a good time for Senseonics, which ended the first quarter with about $13 million in cash and marketable securities, and has been burning through about $11 million a quarter, according to BTIG analyst Sean Lavin. The new funds should help the company at least get through the FDA process, hopefully by the end of the year, Lavin noted in his report.
Roche's participation in the offering can be seen as a big positive, he added, as the investment shows increased confidence in the product's merits.
During Senseonics' first quarter earnings call, CEO Tim Goodnow characterized the company's discussions with FDA as "collaborative and productive," and that a panel meeting can be expected as part of the review process.
Goodnow also talked during the call about the company's efforts to gain a CE mark amendment to extend its sensor life labeling for up to 180 days. That version of the device will be marketed as Eversense XL, he said, and could be rolled out in Europe as early as the third quarter.
"As you can imagine, the combination of an even longer term sensor that can provide continuous readings for up to 180 days will show rapid evolution of the product, and having this coupled with the slim gen 2 transmitter will create yet another level of convenience for our users," Goodnow said.
He also noted that the company is expanding commercialization in Germany from a controlled launch to a full launch, based on positive clinician and patient reception of the technology. That means Roche will be increasing its footprints from four reps to its entire team of intensive diabetes sales reps to cover all of Germany, Goodnow said.
The company's stock (NYSE: SENS) was up 20.57% to $1.70 at 12:40 p.m. EDT on Friday, with trading volume well above average.
Amanda Pedersen is Qmed's news editor. Contact her at [email protected].
[Image credit: Pixabay and Senseonics]
About the Author(s)
You May Also Like