How the ISO 9001:2015 Revision Affects Medical Device Companies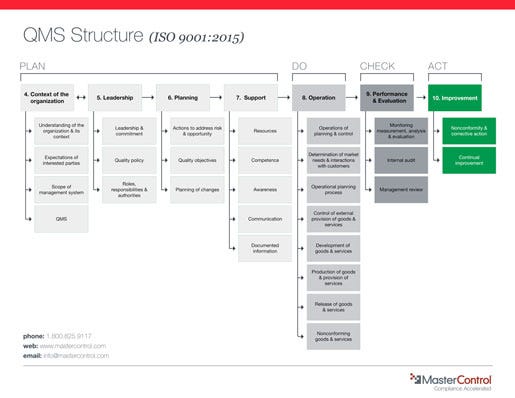
Testing
How the ISO 9001:2015 Revision Affects Medical Device CompaniesHow the ISO 9001:2015 Revision Affects Medical Device Companies
The 2015 revision of the ISO 9001 quality standard contains fundamental changes to the structure and contents of the standard and offers a preview of some revisions to ISO 13485 expected in the coming year.
Sign up for the QMED & MD+DI Daily newsletter.