After recalling the HawkOne Directional Atherectomy System earlier this year, Medtronic is also recalling (correcting) its TurboHawk Plus Directional Atherectomy System due to design similarities with the HawkOne.
March 9, 2022

Editor's note: In an earlier version of this story, TurboHawk was misspelled. MD+DI regrets the error.
Earlier this year, Medtronic recalled its HawkOne Directional Atherectomy System while stopping short of asking for the product to be returned or discarded. Now, the company is issuing a similar recall for its TurboHawk Plus Directional Atherectomy System because of design similarities with the HawkOne device. Again, Medtronic is not asking for product to be returned or discarded.
As with the HawkOne, there is a risk of the guidewire within the Medtronic TurboHawk catheter moving downward or prolapsing when force is applied during use, according to FDA's notice on Wednesday. If this happens, the catheter tip may break off or separate and this could lead to serious adverse events including a tear along the inside wall of an artery (arterial dissection), a rupture or breakage of an artery (arterial rupture), decrease in blood flow to a part of the body because of a blocked artery (ischemia), and/or blood vessel complications that could require surgical repair and additional procedures to capture and remove the detached and/or migrated (embolized) tip.
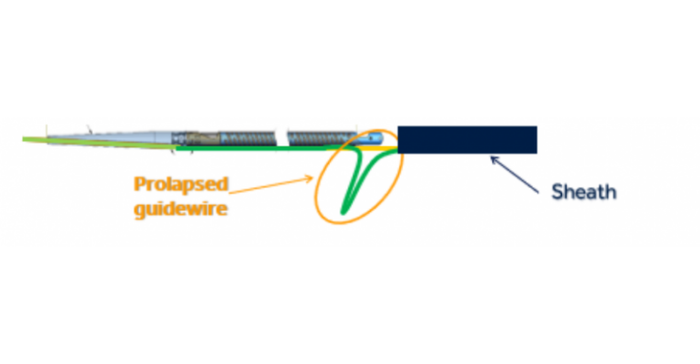
As of Feb. 7, there had been no reported injuries or deaths associated with this problem for the TurboHawk device. The device is used during procedures intended to remove blockage from peripheral arteries and improve blood flow. There have been 163 complaints, including 55 injuries, about the HawkOne device, however.
The TurboHawk recall notice applies to 686 devices on the U.S. market distributed between Sept. 27, 2021 and Jan. 25, 2022.
Medtronic is asking customers to review the instructions for use included with the TurboHawk device, noting the warnings and precautions listed in a letter sent to customers last month. Customers are also being asked to complete a customer confirmation form that was included with the urgent medical device notice.
About the Author(s)
You May Also Like




