How would you rate your level of concern about the following areas, as they relate to the medical design and manufacturing industry? (1=Not at all concerned, 4=Somewhat concerned, 7=Very concerned)
March 15, 2012
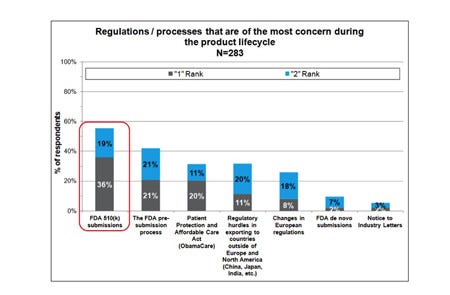
Which regulation / processes are of the most concern to you and your company during the product life-cycle? (Please select the top two in order were 1 = Most significant concern, 2 = Second most significant concern.)
Methodology:
A targeted Internet survey was fielded from February 1st – February 10th, 2012.
Sample:
The sample consisted of 283 respondents from UBM Canon’s database of medical device professionals who replied to an editorial asking for feedback.
Respondent Details:
Over 80% of respondents are male and nearly two-thirds are between 45 and 55 years old.
Half of respondents have Master’s or Doctorate degrees, and nearly half work in a company that employs fewer than 100 people.
One-quarter of respondents work in management and 22% are an owner / partner in their organization.
40% of respondents have at least 20 years of experience in the medical design and manufacturing industry.
You May Also Like




