Medtronic is recalling the pump implant kit for its HeartWare Ventricular Assist Device two deaths and many serious injuries were reported. This is the company's second HeartWare-related recall in about a year, and one of several in the device's history.
March 3, 2021
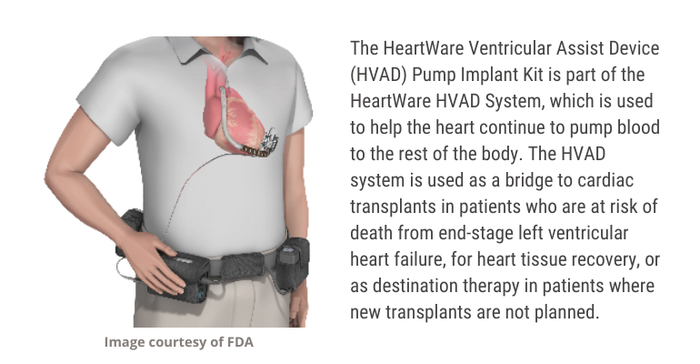
Medtronic is recalling the pump implant kit for its HeartWare Ventricular Assist Device (HVAD) because the device may fail to initially start, restart, or have a delay in restarting after the pump stops, according to a FDA MedWatch notice issued Tuesday.
This latest HeartWare issue (failure to start or restart) has occurred during preimplant testing, during the implant, or in a variety of post-implant situations. There have been 29 complaints about this device issue, which include 19 serious injuries and 8 cases of patients who had a life-threatening event but recovered without long term effects. Two deaths have also been reported.
The HVAD Pump Implant Kit is part of the HeartWare HVAD System, which Medtronic inherited through its $1.1 billion acquisition of HeartWare in 2016. According to the recall report, 157 devices are included in this recall.
While the issue was just flagged this week in the FDA MedWatch notice, Medtronic initiated the recall last November, and sent urgent letters to customers in December advising healthcare providers on following the current instructions of use to avoid unnecessary pump stops. Most of these instructions pointed out by the company have to do with not disconnecting the driveline from the controller, and not disconnecting both power sources at the same time, but it also suggests that in some cases the patient might have to have the controller exchanged. If a controller exchange is necessary, it should be done under clinician supervision in a controlled environment with immediate ability to put the patient on hemodynamic support, the notice said.
HeartWare has a history of trouble
In May 2020, Medtronic recalled the HeartWare HVAD pump outflow graft and outflow graft strain relief because of the risk of breaks and tears during the pre-implant pump assembly process. About two years prior, the company had a recall that included 204,017 of its devices. The issue stemmed from an interruption of the electrical connection between the device’s power source and the HVAD controller.
There were also two significant recalls involving the HVAD just before after Medtronic acquired HeartWare in 2016. One involved 8,799 HVAD controllers that were potentially damaged from exposure to moisture through loose power and data connectors. The other involved 350 unimplanted kits sitting in hospital inventory that were susceptible to electrical faults and connection failures from fluid entering the driveline-to-controller connector.
HeartWare had a major battery recall involving more than 18,000 HVAD batteries sold between 2013 and 2015, also prior to Medtronic buying the company.
About the Author(s)
You May Also Like




