The ability to diagnose at a point-of-care setting will help with more quickly and appropriately treating sexually-transmitted infections, expert says.
March 31, 2021
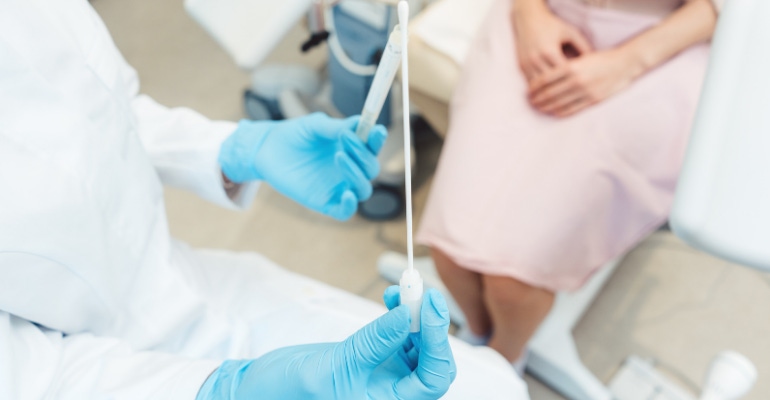
FDA said Tuesday that it will allow the use of the Binx Health IO CT/NG Assay at point-of-care settings, such as in physician offices, community-based clinics, urgent care settings, outpatient healthcare facilities and other patient care settings. The test, which uses female vaginal swabs and male urine specimens, is designed to detect the presence of the bacteria Chlamydia trachomatis and Neisseria gonorrhoeae, which cause the sexually transmitted infections chlamydia and gonorrhea, respectively.
FDA said this action is the result of the agency granting a waiver under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) for the Binx Health IO CT/NG Assay.
“The ability to diagnose at a point-of-care setting will help with more quickly and appropriately treating sexually-transmitted infections, which is a major milestone in helping patients,” said Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in FDA’s Center for Devices and Radiological Health. “More convenient testing with quicker results can help patients get access to the most appropriate treatment. According to the CDC, one in five Americans are diagnosed with sexually transmitted infections every year, which is why access to faster diagnostic results and faster, more appropriate treatments will make significant strides in combatting these infections.”
Following clearance for use by individuals trained in the test procedure and under the oversight of a CLIA certified laboratory that meets the requirements for high or moderate complexity testing, Binx Health applied for a CLIA waiver based on data demonstrating that the test meets the criteria for such a waiver.
The test is performed while the patient is present, providing an actionable result in about 30 minutes. The test’s performance was evaluated in females 16 years and older and in males 17 years and older and was demonstrated to be comparable to tests performed in a CLIA certified laboratory that meets the requirements for high or moderate complexity testing.
About the Author(s)
You May Also Like


