In the debate on medical device regulation, there has been a great deal written and said—on all sides of the debate—about whether the current 510(k) program stifles medical device innovation. It isn’t clear to me, however, what exactly is being discussed The administrative premarket notification [510(k)] program is not used to regulate what the public generally considers to be innovative medical devices; that is the purpose of the premarket approval (PMA) program. So why is the 510(k) program so often mentioned in this context?
August 10, 2012
In the debate on medical device regulation, there has been a great deal written and said—on all sides—about whether the current 510(k) program stifles medical device innovation. It isn’t clear to me, however, what exactly is being discussed. The administrative premarket notification [510(k)] program is not used to regulate what the public generally considers to be innovative medical devices; that is the purpose of the premarket approval (PMA) program. So why is the 510(k) program so often mentioned in this context?
Let us first define “innovation.” It is not a loosely-defined term and its meaning is not discretionary; it is quite specific and has been so in science, technology, and business for over the past half century (see E. Rogers, 1962-20031; E. Mansfield, 19682; and many others). A rational public debate on policy requires precise language. Innovation is not synonymous with invention! Innovation is the dissemination (often by commercialization) of an invention after it has been developed into a viable product, process, or service. Innovation presupposes invention, but is not invention.
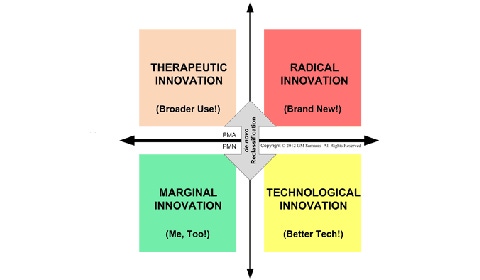
The U.S. regulatory process for innovative medical devices can be depicted, in a simplified form, by modifying the Henderson-Clark model of product innovation from 1990 (see Figure 1). In the case of U.S. regulation of medical device marketing, regulation is strongly dependent on two dimensions of innovation: technology and intended use.
Figure 1: Medical device innovation and U.S. regulation. Image copyright G.M. Samaras. |
Medical devices that use essentially existing medical technology for a new intended use, such as ophthalmic surgical lasers, are therapeutic innovations. They have a high level of uncertainty regarding establishing reasonable safety and effectiveness. They are regulated by the PMA program.
Medical devices that are clones of existing, legally marketed medical devices are marginal innovations. An example of such a product would be a new transcutaneous electrical nerve stimulator that is substantially equivalent to another product available on the U.S. market. Such products have been dubbed “me, too” devices and offer very little that is new. If they have essentially the same technological characteristics as their claimed predicate3, they have a very low level of uncertainty regarding how to establish reasonable safety and effectiveness. They are regulated by the 510(k) program.
Medical devices that use new technology (for instance, a change in materials, energy source, hardware, software, and/or human factors design, etc.4) to accomplish the same medical purpose are technological innovations. When they have low uncertainty regarding establishing reasonable safety and effectiveness, they also are regulated by the 510(k) program. Otherwise, they are automatically reclassified and regulated by the PMA program.
So, what about the argument that the 510(k) program stifles innovation? We are excluding from this discussion innovative business models (such as medical devices that are part product and part service), innovative marketing (such as direct sales to patients), and innovative manufacturing (such as molding rather than machining) of medical devices. Also, we are not talking about the PMA program, which requires FDA-supervised clinical trials, extensive data analyses by FDA biostatisticians, and detailed audits of design and manufacturing processes. That process takes considerable time, but we are obliged to balance that against practitioners and patients getting devices that are reasonably safe and effective. In return, federal preemption shields the manufacturer. I have been involved on both sides and, yes, it can be ponderous, but that is not the 510(k) program.
When you discuss innovation in the context of the 510(k) program, you really are referring to technological innovations. Marginal innovations are technically considered “innovations” in academic circles, but not by the public. Technological innovation is like going from the old clunky portable phones to today’s sleek new cell phones (you can call and talk on both); it is typically an incremental process, which may decrease cost, increase reliability, and so forth. Marginal innovation is making a clone; it is not a significant improvement in any real sense.
For the 510(k) program, the issue boils down to figuring out how to do the testing necessary to support your claim that there is low or very low uncertainty about establishing reasonable safety and effectiveness for the already established intended use. This often means recognizing what consensus standards and engineering best practices apply to your particular device. Some are “horizontal” (such as electrical safety, biocompatibility, software development, and human factors issues) and some are “vertical” (they only apply to a specific type of device). In other cases, it means recognizing that there exist particular special controls (“guidance documents”); all special controls are vertical standards. In rare cases, it also means doing a pivotal clinical trial5, because bench testing and animal testing are insufficient.
To avoid wasting both your time and the regulator’s time, you must fully understand both the applicable consensus standards and any special controls and then do the required testing correctly and completely. You do not need to figure this out on your own; you can have a third party testing laboratory do the testing for you or you can have an expert consultant help you do the testing. If you do that completely and correctly from the outset, and present it clearly in your submission, it has been my personal experience that you rarely encounter delays. If you can’t or won’t, there is a very good chance you actually will get “stifled.” Some may say something like: “well, that is not my experience.” But before making that judgment, make sure you are not comparing apples and oranges. If it is complete and correct and it is clearly presented, there is no justification for additional information (AI); if not, expect an AI letter.
References
Rogers E.M. Diffusion of Innovation. 5th ed., Free Press. NY. 2003
Mansfield E. Industrial Research and Technological Innovation: An Econometric Analysis. Norton. NY. 1968
21 CFR 807.100(b)(2)(i)
21 CFR 807.100(b)(2)(ii)(A)
Draft Guidance for Industry, Clinical Investigators, and Food and Drug Administration Staff - Design Considerations for Pivotal Clinical Investigations for Medical Devices. August 15, 2011.
G.M. Samaras is a biomedical scientist and engineer in private practice (Pueblo, CO). Trained as an electrical engineer, he has doctorates in physiology and industrial engineering; he is a licensed professional engineer, board-certified human factors engineer, and an ASQ-certified quality engineer. He has a number of biomedical patents and publications in physiology and engineering (hardware, software, human factors, and quality). He has worked at the FDA/CDRH as a reviewer and manager, he was a medical school and engineering graduate school professor, and founded an engineering firm that he ran for a decade.
About the Author(s)
You May Also Like


