There have been 27 complaints about this device issue, 26 reports of serious injuries, and one death has been reported.
February 3, 2021
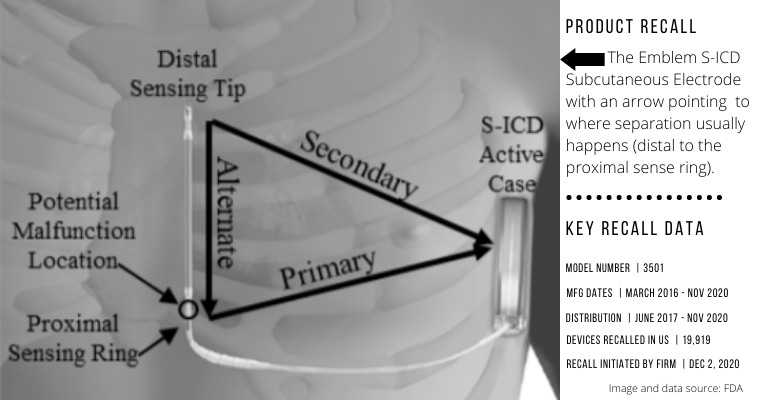
Boston Scientific is recalling the Emblem S-ICD Subcutaneous Electrode because of increased risk of fractures at a specific point (distal to the proximal sense ring), FDA reported this week. The company initiated the recall in December.
If the device fractures during use, it could become unable to deliver therapy to slow very fast heartbeats from cardiac arrest (tachycardia). A failed device may cause serious adverse events, the agency said. Examples include injury or death if cardiac arrest cannot be treated or need for additional surgery to replace failed devices.
So far, FDA said, there have been 27 complaints about this device issue and 26 reports of serious injuries associated with this recall. One death has also been reported.
The Emblem S-ICD Subcutaneous Electrode is part of the Boston Scientific S-ICD System. The Boston Scientific S-ICD is an implantable cardioverter defibrillator that is intended to provide pacing for slow heart rhythms and electrical shock or pacing to stop dangerously fast heart rhythms. These cardiac devices are implanted under the skin in the upper chest area.
The company reported strong safety and efficacy data on the Emblem S-ICD in 2019.
A tale of another Boston Scientific S-ICD safety issue
Back in 2017, Boston Scientific had to develop an S-ICD software update after a super rare malfunction caused a device-related patient death. In that incident, the patient died after the device repeatedly delivered an atypical amount of energy because a specific memory location was corrupted by radiation within the environment. This repeated atypical energy delivery prevented an arrhythmia detection and subsequent treatment, and ultimately contributed to the patient's death, the company said.
The probability of the same thing happening to another patient is 1 in 300,000 over five years, Boston Scientific determined after its investigation of the 2017 incident. Still, the company developed software updates for its Emblem S-ICDs and SQ-RX S-ICDs as a precaution. This problem cannot occur with any of the company's transvenous defibrillators or pacemakers because of hardware and software differences, Boston Scientific noted at the time.
Engineers were able to simulate the device behavior in a lab by corrupting two specific adjacent bits of device memory on similar model S-ICDs. The software update would mitigate the effects of memory corruption by preventing atypical energy delivery, Boston Scientific said.
The company said the memory corruption that happened in the reported case was due to a transient change of the device's operating state, caused by what engineers refer to as a single event upset (SEU). An SEU is a change of state in the device memory induced by environmental radiation interacting with a specific memory location.
All electronic devices that use integrated circuits are susceptible to SEUs, but implantable heart devices include mechanisms that are supposed to detect and correct memory corruption before it interferes with the device's function. This type of corruption can't always be detected though, especially if it impacts multiple bits in an area of memory that is expected to change as software performs device operations. That's what happened in this case, Boston Scientific said.
The company also noted that the patient had not been subjected to ionizing radiation therapy or any other readily identifiable external source of ionized particles prior to the incident.
Recommendations for physicians and patients impacted by the current recall
Boston Scientific has made the following recommendations to help physicians and patients evaluate the risks of using affected devices compared to replacing them.
Enroll and monitor patients through Latitude remote monitoring to detect any alerts or artifacts on the devices in between office device checks. Ask patients to do weekly remote checks.
Perform a system follow-up every three months by remote or in-office checks. Assess sensing performance in-clinic if any of the following is observed:
Non-physiologic, mechanical artifacts (if artifacts are found, this may be a sign of an electrode body fracture)
High electrode impedance alerts
Capture all sensing vectors that may indicate onset of electrode body fracture
Review stored devices for artifacts, as this may be a sign of potential electrode body fracture
Perform a chest radiography of the entire electrode length if there is a suspicion of a break. In the absence of any indications of electrode fracture, surveillance x-rays are not recommended.
Demonstrate the device beeper to the patient during the next office visit. For patients not monitored by Latitude, repeat the beeper demonstration following any MRI scan, as strong magnetic fields may cause permanent loss of beeper volume. Remind all patients to quickly contact their physician if beeping tones are heard from their device or if a shock is delivered.
Evaluate the risk for life-threatening harm due to an electrode body fracture. This is greatest for patients who:
Have a history of life-threatening ventricular arrhythmias such as secondary prevention
Are unable to be reliably followed remotely or in person every three months
Are not monitored via Latitude and are unable to hear beeping tones
Replace any electrode that is shown to have breaks with signs like artifacts, high impedance alert, or x-ray results. Routine replacement of an electrode without evidence of breaking is not recommended. Return explanted devices to Boston Scientific.
About the Author(s)
You May Also Like




