Originally Published MDDI July 2006 COVER STORY: NANOTECHNOLOGYThe field of nanotechnology is exciting, vast, and maybe a bit confusing. Device makers may need to understand the minutiae and the market before venturing into the nanoworld.
July 1, 2006
COVER STORY: NANOTECHNOLOGY
|
A nanotube with H2 molecules. A nanotube is about half the diameter of a DNA molecule. Image courtesy of Nanomix (Emeryville, CA). |
Over the last few years, the term nanotechnology has popped up increasingly in the media. For the medical device industry, the term is being woven into discussions of sensors, coatings, and the cutting edge of R&D. But the term is broad, and it is often difficult to identify what it means in real terms. These nanowires, nanotubes, and nanoparticles are the tiniest of structures. Nanotechnology-based products generally encompass materials up to 100 nanometers, but according to nanotechnology experts, it's what a nano-based product does rather than its size that really matters.
“Nanotechnology is not necessarily a size range, but actually it's the point at which emergent properties take over to offer performance benefits,” explains David Lackner, an analyst for Lux Research (New York City). Lux specializes in market intelligence on nanotechnology. “Nanotechnology gives you properties that you wouldn't have at any other scale.” Lackner provides two key examples—aluminum and silver—one of which is already making some headway in the medical device industry. Silver, which is being used in wound dressings and as a coating, becomes antimicrobial at the nanoscale level.
“Active research is taking place, but some researchers still view nanotechnology and nanorobotics as merely a fantasy,” says Sangeetha Prabakar, an analyst for Frost & Sullivan. “However, nanorobotics seems to be a fast-growing industry with tremendous future potential, which, in spite of its criticisms, is likely to develop into a powerful field.”
Other experts agree. “Saying ‘nanotechnology' is really just saying ‘cutting edge.' It's not a field, and it's not an industry. It's really a metafield, combining academic research and industrial development,” says Lynn Foster, emerging technologies director at Greenberg Traurig in Santa Monica, CA. In his book, Nanotechnology: Science, Innovation, and Opportunity, Foster notes that nanotechnology “will accumulate incrementally, in small cascades over decades rather than in a sudden engulfing wave of change.”
For the medical device industry, certain nano-based developments have applications now, and others are not too far off in the future. A few still sound like science fiction. It is critical to understand the key challenges, potential applications, and regulatory issues to make the most of nanotech developments—current and future.
From Research to Reality
|
Buckminsterfullerene (C60) balls are shown inside a carbon nanotube. The colored peaks show electron waves. The nanotube is a cylinder of carbon atoms less than a billionth of the width of a human hair. Image by David Luzzi, University of Pennsylvania/Photo Researchers. |
Governments, industry, and academia worldwide are discussing, defining, and refining nanotechnology to uncover its possibilities. In the United States, the National Nanotech Initiative (NNI) is a collaborative program that involves multiple agencies, including the National Institutes of Health, HHS (including FDA), the National Science Foundation, and the U.S. Department of Commerce, just to name a few. The initiative is designed to foster interagency efforts to explore all things nano.
“The government has done a really good job of funding basic science, and the academic community is doing tremendous research work, so out of that a natural progression will be innovation that can be commercialized,” says Foster.
In terms of commercialization, though, it's been a bit of a slow transition. “For most industries, companies developing nanotechnology applications have found few commercial successes in their university collaborations,” says Lackner. “Many corporate planners are putting the pinch on academic partners to show a return on investment in less than five years.”
The good news, he says, is that medical device companies have more experience, nano or not, in taking university-based research and doing something with it. Smith & Nephew, for example, is already making and selling an antimicrobial bandage using silver nanoparticles. Nanomix and AcryMed are other examples of companies that are already commercializing products for the medical device industry. He notes that Guidant, now owned by Boston Scientific, and Medtronic are looking at antimicrobial coatings for their devices, and that Guidant is exploring nanotechnology to improve electrodes in its products.
Achieving market adoption and overcoming regulatory hurdles are key to the integration of nanotechnology in more devices. “Device companies are willing to address legal and FDA issues related to nanotechnology to get products to market. This indicates something about their level of acceptance of nanotechnology,” he says.
For companies that are struggling with the challenges of exploring nanotechnology options, Lackner provides some advice. “With nanotechnology, smart companies affiliate themselves with universities early in the process. They secure access to future IP and to the people involved. They also set a specific timeline, set milestones, and hold to them,” he advises.
The Future of Nanomedicine
|
The charge density of hydrogen on a single boron-nitride (BN) sheet. Image courtesy of Nanomix (Emeryville, CA). |
In December 2005, the European Science Foundation released a 48-page report on its two-year study of the applications for nanotechnology in healthcare. The report, “Nanomedicine,” set out not only to define nanotechnology but also to examine its future effects on healthcare and society.
The report defines the field of nanomedicine as “the science and technology of diagnosing, treating, and preventing disease and traumatic injury, of relieving pain, and of preserving and improving human health using molecular tools and molecular knowledge of the human body.” It divides nanomedicine into the following four main subdisciplines:
• Analytical tools.
• Nanoimaging.
• Nanomaterials and nanodevices.
• Novel therapeutics and drug-delivery systems.
The report notes that these disciplines share common technical issues, including clinical, regulatory, and toxicological issues. The report stresses that to realize nanomedicine's full potential in the future, new regulatory guidelines must be developed soon to ensure safe and reliable transfer of advances in technology to reality.
“Companies that are ahead of the curve are the ones developing products now,” says Peter Gluck, an attorney with Greenberg Traurig's Costa Mesa, CA, office. But he also says that it will be three to five years before the first big wave of nanotechnology-based medical products are on the market. “By 2010, we will have already gone through the paradigm shift. Around 2007 or 2008, we will start seeing the first wave of nano-based devices, and in 2009 they'll be out there generally,” says Gluck.
|
Stacked nanotubes may be used in point-of-care diagnostics and monitoring applications. |
This wave of technology, he says, is going to bridge the gap between traditional in vitro and in vivo analyses. “What we're going to see is faster analysis, all onboard a single device that can be transported, say, to a battlefield.”
“Nanotechnology developments are enhancing traditional areas. Nanotechnology expands the uses for different materials in implants,” says Foster.
Gluck notes that companies are now working on smart prosthetics using nanoscale materials. “These materials allow better conductivity. You can have embedded chip sets in a prosthetic with sensing technologies. It accomplishes tasks that took three or four different kinds of devices in the past,” says Gluck. “We're working with a couple of companies that are looking at putting the devices onto and into people now within a six-month window.”
Just deciding to explore nanotechnology isn't enough, however. “First and foremost, companies must pick the right applications,” advises David Macdonald, president and CEO of Nanomix (Emeryville, CA). “We focused on picking high-value applications in which critical information is needed right away, such as in an emergency situation to make an immediate assessment or decision.” He also advises making the products perform in a way that will provide reliable information at a reasonable cost.
The World's Tiniest Robots
Sidebar: |
Nanorobots may sound like science fiction, but these tiny structures have applications as both medical devices and as diagnostics. Because of their size, they are creating new territories for devices. As new products are developed, these devices may create a whole new class of products, particularly for the purpose of submitting them to FDA.
Nanorobots are a kind of nanodevice, explains Prabakar. She notes that there are several types of nanodevices, including nanopores, nanotubes, microscopic molecular dots or quantum dots, nanorods, nanoshells, and dendrimers. The sidebar, “Pores, Tubes, Dots, and More” provides a list of definitions.
|
A scanning electron microscope (SEM) focuses in on nanoturf, or aligned carbon nanotubes. Image courtesy of Nanomix (Emeryville, CA). |
“Nanorobots are minuscule, resolute automatons that will be used for maintaining and protecting the human body against pathogens,” explains Prabakar. “A nanorobot has a diameter of about 0.5–3 µm. It is constructed from parts with dimensions ranging from 1 to 100 nm.” Such nanorobots, she says, will be capable of assembling structures made up of between 100 and 10,000 components and may measure tens of microns. The main element used in a nanorobot is carbon in the form of diamond and fullerene nanocomposites. Carbon is preferred in this form, she says, because of its strength and chemical inertness.
Researchers have already constructed nanomotors called assemblers. A nanotechnology company called Zyvex is developing a device with the goal of building the key tool for creating molecular nanotechnology, the assembler. According to Zyvex, the first assembler could be available within the next few years, but Prabakar expects it to be some time before medical nanorobots reach the market.
|
An SEM view of nanoparticles forming nanograpes. Image courtesy of Nanomix (Emeryville, CA). |
She predicts an integrated system will be developed by the year 2015, with many more commercial healthcare applications to be available by 2020.
“With tremendous research pursued by expert scientists in different parts of the globe in the area of nanodevices, the scientific community is aiming to develop nanodevices that do much more than deliver treatment,” says Prabakar. She believes that quantum dots, nanopores, and other devices for detection and diagnosis may be available for use in 5–15 years.
Specially designed nanorobots may fulfill important new roles in the future, particularly for the biomedical industry. For example, she says that noteworthy technology developments such as nanowalkers are being developed at the Massachusetts Institute of Technology in Cambridge, MA. “Nanowalkers are expected to navigate through the body to image and diagnose diseases that are difficult to diagnose using conventional scanning techniques. Such devices will also deliver drugs to tumors and repair organs without surgery.”
|
An SEM view of boron-nitride nanotubes growing from a seeding particle. Image courtesy of Nanomix (Emeryville, CA). |
Another important application, she says, are nanorobots for cell repair. Such nanorobots will be injected into the patient's body. The robots will then patrol the body searching for damaged cells. These robots will restore normal function to cells and even restore dead cells as long as a cell's original structure is partially intact. They will also detect and destroy disease-causing viruses and could be used to detect DNA mutations.
“There are a lot of different applications being used for sensing, and there's a tremendous body of research for things like early cancer detection. For example, companies like Nanomix are using carbon nanotubes,” says Foster. “There are also developments using nanoparticles to make coatings that will make implants more biocompatible.”
Some applications, says Foster, are still a decade away from commercialization. He expects diagnostic applications involving detection of cancer consisting of just a few cells in size will be a reality in 5–10 years.
There are also long-term developments like implantable batteries that would take energy from glucose, he says. “You could have pacemaker batteries that never have to be replaced. The batteries would function as long as the pacemaker is there.” Such developments, however, are likely to be at least five years away.
Many drug-delivery applications are also extremely promising. Foster predicts that nanotechnology will significantly affect these applications. He points to companies like Insert Therapeutics (Pasadena, CA). The company's technology platform is based on nanoscale materials that use modified cyclodextrin molecules as building blocks to create a new class of proprietary drug-delivery materials called linear cyclodextrin-containing polymers. According to the company, its Cyclosert forms nanoscale constructs with hydrodynamic diameters between 30 and 60 nm.
“You can target cancer cells much faster and much more directly so you're not allowing a huge amount of something that's poorly soluble to float around in the body causing side effects,” says Foster.
“Some of the traditional distinctions between in vivo and in vitro can be overcome because we're using nanodevices to fill the gap,” says Gluck. “For example, we have a lot of clients that have taken larger-scale diagnostic systems and put them into nanoscale devices—the lab-on-a-chip idea. They can now do on a chip what took a lot longer and required an entirely outfitted lab space before. We're seeing a lot of improvements and progress based on using the nanotools to perform the same function.”
No Bigger Than a Pupil
“There's no question in my mind that nanomaterials will be the future platform for point-of-care diagnostics and monitoring applications,” says Macdonald. Nanomix has developed a portfolio of handheld sensors for detection of gases and biomolecules. The company plans to submit its Emergency Respiratory Monitoring device for FDA clearance by the end of this year.
According to Macdonald, these materials have always been around, but the tools for manipulating and imaging them have become much better over recent years. Imaging systems such as scanning electron microscopes, tunneling electron microscopes, and atomic force microscopes have become much more affordable and more sophisticated.
“Nanotechnology has been in research mode and really still is, with the exception of a handful of companies that are completely product driven. For example, we are focused on point-of-care medical diagnostics and monitoring applications,” explains Macdonald. “We've chosen a fairly specific group of analytes that we think can add the most value with our detection platform. The majority of our applications are going to be in the form of point-of-care medical devices for diagnostics and monitoring.”
When considering the health-related concerns with nanomaterials, it's important to separate in vitro applications from in vivo applications, he notes. “There is quite a bit of ongoing targeted drug-delivery research work on nanomaterials that will locate a tumor and deliver drugs selectively to the tumor cells,” he says. “There is a lot of promise for these types of applications. For in vivo applications where they're actually injecting nanomaterials into the body, there needs to be adequate study of health-related concerns.”
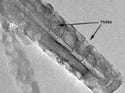
|
A transmission electron microscope (TEM) view of a closed end of a multiwall nanotube with induced defects (holes). Image courtesy of Nanomix (Emeryville, CA). |
“I believe we should expect in vitro products to appear in a shorter time frame than in vivo products because in vitro products are lower risk and won't require the same degree of study as to long-term effects,” says Macdonald.
Nanomix applications use an external sample (saliva, blood, etc.) to conduct a test using encapsulated nanomaterials. “The nanomaterials we use do not enter the body. They're fully encapsulated, and the body doesn't come in contact with them,” he says.
|
A multiwall carbon nanotube receives its seeding particle. Image courtesy of Nanomix (Emeryville, CA). |
The Nanomix Sensation detection platform is an in vitro technology using random networks of carbon nanotubes treated with special recognition chemistries. “We make very sensitive and specific detection devices with several unique features. Carbon nanotubes are very small (1–2 nm in diameter) and consist of a combination of metallic and semiconductor materials. By applying very small amounts of electric current, we can measure a very small change in the electronic characteristics of these devices,” he says.
Macdonald says the biggest advantage of the company's detection platform is that it provides label-free detection. Many traditional diagnostic methods are based on optical detection techniques. These techniques use expensive labeling chemistry that emits either a fluorescence or chemiluminescence signal, captured and quantified by sophisticated equipment needed to turn light emission into an actual result. “We eliminate that step by using nanoelectronic detection,” explains Macdonald. “These devices detect and quantify a variety of gases and biomolecules directly and electronically. It's a nonoptical direct-detection mechanism.
Nanomaterials work well for biomolecule detection, he says, because of their size compatibility. “Carbon nanotubes are roughly the same size as a DNA strand. This allows for ultrasensitive direct detection of these molecules,” says Macdonald. “With Sensation technology, the solid phase is the same size as a single DNA capture strand. The binding event is measured directly through an electronic device response. In general, the size compatibility between nanomaterials and biomolecules enables incredible levels of sensitivity that are difficult to obtain with optical detection methods.”
Macdonald says the company's products will be used primarily for point-of-care testing, where devices need to be simple, fast, and inexpensive. He says that it's important for such devices to be scalable. The company uses mature semiconductor processes to produce device arrays by the thousands. “We produce small device arrays—about the size of the pupil of your eye—that contain multiple detection devices. We get thousands of arrays from each wafer, and the process is highly automated,” he says.
“It's important that device manufacturers understand the unique features you can build into products by using nanomaterials,” explains Macdonald. Nanomaterials offer simplified detection equipment, low power consumption, small size, high sensitivity, and so on.
Nano-Based Coatings
|
AcryMed's founder Bruce Gibbins, PhD, (right) and senior research scientist Balu Karandikar compare catheters before (white) and after treatment with silver nanoparticles. Image courtesy of Acrymed (Beaverton, OR) |
The use of nanotechnology to develop medical coatings has already produced commercialized products. In December 2005, I-Flow Corp. (Lake Forest, CA) received FDA clearance for the company's ON-Q SilverSoaker regional anesthesia delivery catheters. The catheters are treated with SilvaGard, a nanotechnology-based silver coating made by AcryMed (Beaverton, OR). The nano-based silver makes the devices impervious to infection-causing bacteria.
“Our particles in the SilvaGard process are approximately 10 nm in diameter. As it turns out, the method produces very uniform particles,” says Jack McMaken, president and CEO of AcryMed. He says that there is a need for the particles to be uniform within certain ranges. “I don't think we would want to have nanoparticles that range between 2 and 99 nm.” Their uniformity enables them to perform more predictably.
“We didn't set out to develop nanoparticle treatments for devices,” says McMaken. “We set out to develop a small amount of silver treatment that had the antimicrobial properties we wanted. As it turns out, we do that through building silver particles on the surface of the devices. Nanotechnology is simply a size that facilitates what we're trying to do.”
In terms of development, McMaken says that applying the coating to the SilverSoaker was a collaborative process. “We asked I-Flow what parts of the catheter they wanted to treat. As it turns out, it was all of them—inside, outside, holes, the whole thing,” he says. Other questions, he says, included how long does the device need to last in the body, what kind of conditions will it see in terms of sterilization, and what types of tissues will it be exposed to.
“So with that kind of information, we were able to tune our process to put the right amount of silver nanoparticles on the surfaces,” explains McMaken. He says that AcryMed did its own testing, but that I-Flow commissioned outside antimicrobial testing as well to confirm that the coating would last as long as the catheter needed it to. It had to be antimicrobial and prevent biofilm formation. However, the silver had to be limited to ensure that it would not be cytotoxic to the tissues it would come into contact with. Because the catheter must mate with other parts, knowing how much the dimensions would change was also important.
“One of the unique features of our technology is that it doesn't change the dimensions of the device,” says McMaken. “The lumen of I-Flow's catheter is 21 thousandths of an inch, and we treat the inside. They insert a diffuser down the length of the catheter (which is 30–35 in. long) and they had to be able to continue to do that.” McMaken explains that AcryMed's process actually builds those nanoparticles into the cracks and crevices of the catheter itself so the nanoparticles don't increase the dimensions of the device.
Another critical question included how long the treatment would be efficacious and not cytotoxic. “We did a lot of elution studies and cytotoxic assays, including some implantation studies. I-Flow also wanted to ensure that the silver nanoparticles didn't change the flex characteristics or the tensile strength of the nylon itself.”
FDA had another question: Even though this is a minuscule amount of silver, some of it comes off. Where does it go? McMaken says the company had “a good feel” for the answer, but didn't have a definitive study. So, the company commissioned a biotracking study at Oregon Health Sciences University.
“You can't just take tissue and analyze it for silver, because you'll always get silver at those low levels,” McMaken says. He says they radio tagged the silver and then implanted the catheter in 6–10 rats. The rats were kept in metabolic cages, and researchers collected urine and feces and measured those with a gamma counter. They then dissected the catheters and the immediate pouch (the tissue around it) containing the catheters and the other major organs. “The amount of silver that this catheter elutes into the body is below the level that already exists in the human body.”
The Regulatory Landscape for Nanotech Devices
FDA has recognized that nanotechnology-based products will present the agency with a number of new challenges. Certainly the unknown risks and limited scientific data make it difficult to address public health concerns. FDA will hold a meeting October 10, 2006, to hear from interested stakeholders about regulatory issues for nanotechnology. FDA has identified a number of issues that it will face with nanotechnology-based products, including the following:
• The agency expects many combination products to emerge.
• FDA may be unaware that nanotechnology is being employed in or on a submitted product.
• The agency must identify standard nomenclature. For example, what is a nanoparticle and what are properties of nanoparticles? Is it the same chemical at a smaller size or does it call for a new formal definition?
• There is limited public health research on the effects of nanomaterials.
One big question that remains, notes Foster, is how to characterize the products that are emerging. “With these hybrid devices, there's a certain point at which they're really a hybrid—they're not a device and they're not a particle—they are really a combination of both,” says Foster. “Government agencies, including FDA, are making progress, but it's moving slowly. The bottom line is that more still needs to be done in terms of definition and characterization.”
Foster says that some of the difficulty lies in the fact that nanotechnology is a part of so many different disciplines. “You have the precautionary principle that you do no harm, but you don't want a blanket answer that wipes out some of the potential health benefits because someone misunderstood, say, the effects of nanoparticles in sunscreen,” he says. “Toxicology, environmental, and safety issues are all key issues that must be addressed.”
If not done carefully, sorting out such details could end up with disastrous results. For example, Macdonald expresses concern that there are already some negative impressions about nanoparticles in general. “There have been environmental health and safety warnings. My concern is that if FDA were to take a very broad action, it would paint us with the same brush as perhaps people using massive amounts of nanoparticles in things like toothpaste and cosmetics, which are intended to go into or on the body,” he explains.
He is also concerned that it could group AcryMed's technology with manufacturing technologies that may use large amounts of nanoparticles. “Manufacturing technologies are so different from our technology because we use very few nanoparticles and you can't get them off the surface of the device. I hope that in FDA's wisdom, it is able to separate those things,” he says.
Even defining nanotechnology-based devices could pose some challenges. “Simply put,” notes Prabakar, “nanorobots are microscopic devices designed to perform a specific task or tasks repeatedly and with precision at nanoscale dimensions.” For the purpose of defining them for FDA, she says, they should be classified as medical devices. However, she adds that it might take a few years to develop commercial nanorobotic devices and, therefore, to determine the class of particular nanorobots based on their safety.
Gluck agrees that nanotechnology is changing devices to the point of affecting their classification. “We're seeing materials now that in the past would have been characterized as Class III implantables, but because they're being done at the nanoscale and being put into the body, they are now something entirely different,” he says. “A lot of our clients are repositioning their FDA strategies to address exactly those issues.”
Regulatory strategies have become much more varied than in the past. Instead of going through the traditional routes, companies may ask FDA to develop a new list of considerations.
Conclusion
Nanotechnology developments are offering medical device manufacturers new properties—and thus new possibilities. The first devices and coatings are starting to appear, and even the long-term outlook for a big wave of development is within the next decade.
Nanotechnology-based devices could revolutionize fields such as cancer detection and point-of-care testing. Nanotechnology-based coatings could be the basis of the next generation of catheters. And, it seems that this is just the beginning.
“We can now work with materials in a way that we never could before,” says Gluck. “It's not so space age anymore. Carbon nanotubes are not far from being ready to be swapped into a lot of the applications that device manufacturers are very familiar with.”
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like