Originally Published MDDI May 2006 NANOTECHNOLOGYOffering the possibilities of smaller, lighter, and faster materials and devices, nanotechnology is working its way into the world of medtech.
May 1, 2006
NANOTECHNOLOGY
|
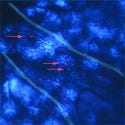
Gold nanorods, which fluoresce red, were photographed inside the blood vessels of a live mouse by researchers at Purdue's Weldon School of Biomedical Engineering and department of chemistry. |
The word nanotechnology was conceived by Norio Taniguchi in 1974 to signify machining with tolerances of less than a micron.1 Since then, nanotechnology has taken on a new meaning. It is now defined as the world of controlling matter at the nanometer (one billionth of a meter) scale.
Nanotechnology is expected to become the transformational technology of this century. Materials fabricated at a nanoscale, i.e., nanomaterials, have different and often amazing properties that can be used to restructure manufacturing, energy production, and a host of other fields.2 Such materials typically have nanostructure-dependent properties (e.g., chemical, mechanical, electrical, biological, optical or magnetic), which make them desirable for medical applications.
Nanotechnology can offer solutions to many current problems by means of smaller, lighter, faster, and better-performing materials, components, and systems. Nanomaterials are being used in computers, cosmetics, stain-resistant fabrics, sports equipment, paints, and medical diagnostic tests. It is essential to understand the definitions that pertain to this new field, the fundamentals of nanomaterials, and current and future medical developments. Equally important to the medical device industry is to understand the risks to health and the regulatory concerns.
Definitions
The definitions of words beginning with nano are not always clear-cut. For example, some descriptions of nanotechnology are not really nano, dealing instead with structures on the micron scale (one millionth of a meter), which is a thousand times larger than a nanometer. In some cases, what is called nanotechnology isn't technology. Rather, it involves basic research on structures having at least one dimension of about one to several hundred nanometers.1 To get a sense of the nanoscale, a human hair measures about a hundred microns across, and a bacterial cell measures a few hundred nanometers. Other definitions include
• Nanomaterials: Materials designed and produced to have structural features with at least one dimension of 100 nm or less.
• Nanoscale: Refers to phenomena that occur on the length scale between 1 and 100 nm.
• Nanoscience: A discipline involving scientific understanding and investigation of nanoscale phenomena.
• Nanostructures: Structures whose characteristic variation in design length is at the nanoscale.
• Nanotechnology: The application of nanoscience in technological devices.
Fundamentals
|
|
These optical microscope images of aligned osteoblast demonstrate the alignment of carbon nanofibers after two days of growth. Source: Purdue University. |
One nanometer is a magical point on the dimensional scale. It is the length of a small molecule. By contrast, atoms measure one-tenth of a nanometer. Nanostructures are at the confluence of the smallest of man-made devices and the largest molecules of living things.1 Nanotechnology is concerned with the shell of the atom, the scale at which the new technology becomes a reality.
Making molecules that can organize themselves on their own or with a supporting surface like metal or plastic is a key strategy for manufacturing nanostructures.2
Nanotechnology and biology share many similarities. The most complicated organisms are made up of tiny cells, which are constructed from nanoscale building blocks: proteins, lipids, nucleic acids, and other complex molecules.3 With nanotechnology, tiny nanostructures are made from semiconductors, metals, plastics, or glass.
At the nanoscale, materials behave differently. Increased reactivity is one such difference; this property is a function of the increased ratio of an object's surface area to its volume as it gets smaller. Silver, for example, performs differently at the nanoscale. The increased reactivity of nanoparticles of silver is used in infection control.
Because each tiny particle in a nanostructure has its own surface area, it increases the overall surface area of silver oxide, which means that more silver can interact in body fluids to encounter and inhibit microbes.4 Gold becomes a good catalyst for fuel cells at nanoscopic sizes and is being used in a number of nanotechnology devices for medical purposes. Nanoscale building blocks fabricated from 1-nm buckyballs made from carbon can be used as a scaffold to repair damaged tissue and bone.1
Nanoparticles can be coated with other substances, allowing materials of such composite particles to combine several properties. One example is employing ceramic nanoparticles with organic shells to reduce the surface tension of water and then using the combination as an antifogging coating.2
Current and Future Developments
|
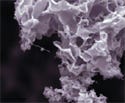
Hydroxyapatite crystal is grown on a coated nanotube surface. Photo supplied by the University of California at Riverside. |
More than 60 drugs and drug-delivery systems based on nanotechnology, and more than 90 medical devices or diagnostic tests, are already being tested, according to NanoBiotech News, a weekly newsletter that tracks advances in the field.5 One device includes the use of quantum dots, bits of material so tiny that they are often just a few atoms across. The dots are used as research tools to help understand how proteins, DNA, and other biological molecules attach to transport systems inside cells. Quantum dots are coated with a material that makes them attach to specific target molecules that may be early indicators of disease.
Clinical studies have begun to study an adaptive retinal implant designed to restore partial vision in cases of blindness caused by retinitis pigmentosa. The system includes a tiny camera in the frame of eyeglasses. The camera transmits images of the surroundings to a special adaptive signal processor.2
Developments in nanoscale biomedicine should be able to create implants that release drugs on demand and monitor blood chemistry. According to the European Commission, with nanotechnology, nanoelectronics, and microsystem technology, complex analysis equipment will become available that will be within the price range of the private household.2 A tiny jab in the finger will be enough for future blood analysis to measure cholesterol and glucose, and the results can be e-mailed to the nearest nanomedical center for a more accurate diagnosis and treatment.
Using nanotechnology, the medication could be carried in supramolecular hollow molecules, nanoscale transport containers with antennas to which antibodies of similar sensory proteins are attached. When the molecules come into contact with structures typical of the agent responsible for the illness, they dock onto it and send a signal to the hollow molecule, which opens up and releases its contents.2 With such nanotechnology, medications could be delivered in high doses directly to the source of the illness, placing no stress on the rest of the body and minimizing side effects.
In the United States, the National Cancer Institute has committed to a new $144.3 million, five-year initiative to develop and apply nanotechnology to cancer. According to the institute's former director and acting FDA commissioner Andrew von Eschenbach, “Nanotechnology has the potential to radically increase our options for prevention, diagnosis, and treatment of cancer.” He added that the institute's commitment to this cancer initiative comes at a critical time and that nanotechnology supports and expands scientific advances in genomics and proteomics while it builds on our understanding of the molecular underpinning of cancer.6
One of the first nanoscale devices to show promise in fighting cancer and administering drugs is a tiny construction called a nanoshell. A nanoshell consists of beads that are about three millionths of an inch wide, with an outer metal wall and an inner silicon core. By varying the size ratio between the wall and core, scientists can tune the shells precisely to absorb or scatter specific wavelengths of light. Gold-encased nanoshells can convert these specific wavelengths of light into heat.
|
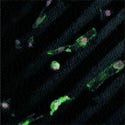
Microcapsules that could deliver drugs are small enough to pass through living cells. Photo courtesy of Rice University. |
Selectively binding these shells to malignant cells could provide a means for fighting cancer. Infrared rays would pass harmlessly through soft tissue but generate lethal heat where they strike the nanoshells. In laboratory tests, investigators have used this selective heating to cook tumor cells without harming surrounding healthy cells.3 Nanoshells may also be able to trigger implanted temperature-sensitive drug-delivery devices, releasing a dose only when illuminated with a specific infrared wavelength.
A key focus of nanotechnology researchers is to develop new ways to seek out and destroy cancer cells. Nanoshells work by cooking cells, but there are other methods. Ralph Weichselbaum, chief of radiation oncology at the University of Chicago, and Vigi Balasubramanian of the Illinois Institute of Technology are collaborating on a project to incorporate a cancer-killer gene into a nanocapsule.
The gene elaborates tumor necrosis factor, which, when injected in large doses, is toxic not only to cancer cells but also to healthy cells. To avoid damage to normal tissue, the nanocapsule is coated with sensors that zero in only on tumor cells. A patient would then be exposed to low-dose radiation or drugs that trigger the gene to make the necrosis factor.7 Nanoparticles are also capable of passing through the blood-brain barrier filter system, so that they can be used as specially coated magnetite particles that are warmed by an alternating electromagnetic field to combat brain tumors.2
Nanosphere Inc. is developing molecular testing systems that would enable detection of patient predisposition to medical conditions and allow physicians to optimize patient drug response based on genetic variations while simultaneously reducing the occurrence of adverse drug reactions.8 The company has developed a system using gold nanoparticles attached to strands of nucleotides complementary to targets of interest such as the mecA gene, a biomarker associated with clinically challenging methicillin-resistant Staphylococcus aureus. When a target nucleic acid or protein is present, the nanoparticle probes latch onto the match and provide an optical signal indicating that the target has been found. The system is ready to adapt to a full range of targets as soon as clinically relevant markers become available.
Risks to Health
Although there are a number of promising breakthroughs in medicine, relatively little is known about the potential health and environmental effects of tiny particles. Millions of dollars are being spent on product development, but some scientists feel that insufficient funds are committed to determining whether nanomaterials pose a danger to human health. It is recognized that subtle changes in the size of the particles used in the nanoscale materials can precipitate widely different changes in their properties, including their toxicity.
Laboratory studies, for example, have shown that inhaled airborne nanoscale materials depositing in the respiratory tract can cause an inflammatory response.9 The small size of engineered nanomaterials also makes their uptake easier into and between various cells, allowing for transport to sensitive target sites in the body, including bone marrow, spleen, heart, and brain. In addition to size, the shape, solubility, surface chemistry, and surface area of ultrafine particles are known to increase inflammation and tissue damage. These are not properties that are usually considered when evaluating hazards and health effects.10
At a recent hearing presented to the U.S. House of Representatives Committee on Science, the director of the Project on Emerging Nanotechnologies, David Rejeski, described the current lack of knowledge about nanotechnology-based products, possible health and environmental implications, and the oversight process designed to manage any potential risks that could breed U.S. public mistrust and suspicion.11 He pointed out that the technology is developing more rapidly than the understanding of the environmental, health, and safety risks, and more rapidly than the government's ability to respond with effective policy measures. Rejeski asked for a coordinated federal strategy, i.e., a nano safety reporting system, and additional funding to address current and future safety concerns, particularly since many nanotechnology-based products, such as cosmetics and consumer products, are entering the market in areas with little or no government oversight.
A recent seminal paper points out the strong likelihood that biological activity of nanoparticles will depend on physiochemical parameters not routinely considered in toxicity screening studies.12 Because engineered nanomaterials show behavior that depends on their physical and chemical structure, risk assessment paradigms that have been developed based on traditional bulk chemistry alone may no longer be valid. This same paper provides a comprehensive set of principles for characterizing the potential human health effects from exposure to nanomaterials. The paper can be used as a screening strategy for risk assessment purposes. Oral, dermal, inhalation, and injection routes of exposure are included, recognizing that, depending on use patterns, exposure to nanomaterials may occur by any of these routes.
Regulatory Issues
According to a recent review, nanotechnology has three major uses in medicine.13 The first is delivering the exact dose of a drug to the intended location. The second is providing new ways to grow and repair body tissues; the third is using the detection of single molecules in diagnosis. All of this means that new discoveries will present many exciting challenges for regulatory affairs, clinical research, and research and development personnel.
Becoming conversant with pharmacodynamics and pharmacokinetics for recently marketed combination drugs and devices, including those on the horizon, requires basic knowledge about physics, chemistry, biochemistry, genetics, molecular biology, materials science (i.e., nanomaterials), toxicology, bioinformatics, and engineering. In addition, the toxicology and environmental effects, as they relate to nanoparticles, will be extremely important issues for manufacturers.
Any of this information that is related to the new delivery system or compound must be compiled, understood, and lucidly explained in writing or, perhaps, verbally to agency reviewers prior to gaining premarket approval.
References
1. M Ratner and D Ratner, Nanotechnology (Upper Saddle River, NJ: Prentice Hall, 2003).
2. Nanotechnology: Innovation for Tomorrow's World (Brussels: European Commission, 2004).
3. AP Alivisatos, “Less Is More in Medicine,” Understanding Nanotechnology (New York: Warner Books, 2002), 56–69.
4. D Tobler and L Warner, “Nanotech Silver Fights Microbes in Medical Devices,” Medical Device & Diagnostic Industry 27, no. 5 (2005): 164–169.
5. R Weiss, “Nanomedicine's Promise Is Anything but Tiny,” Washington Post, January 30, 2005: A08.
6. NIH News, National Institutes of Health, September 13, 2004.
7. R Kotulak, “Tiny Battlefield in the War on Disease,” Chicago Tribune, September 14, 2004: 10.
8. “Technology Overview” [online]; available from Internet: www.nanosphere-inc.com/tech.
9. G Oberdorster et al., “Nanotoxicology: An Emerging Discipline Evolving for Studies of Ultrafine Particles,” Environmental Health Perspectives 113, no. 7 (2005): 823–839.
10. A Maynard and E Kuempel, “Airborne Nanostructured Particles and Occupational Health,” Journal of Nanoparticle Research 7 (2005): 587–614.
11. Testimony of David Rejeski, director, Project of Emerging Nanotechnologies, before the U.S. House of Representatives Committee on Science, November 17, 2005.
12. G Oberdorster et al., “Principles for Characterizing Potential Human Health Effects from Exposure to Nanomaterials: Elements of a Screening Strategy,” Particle and Fibre Toxicology [online] October2005 [cited 5 April 2006]; available from Internet: www.particleandfibretechnology. com/content/2/1/8.
13. T Delamothe, “Nanotechnology: Small Science, Big Deal,” British Medical Journal 330, no. 5 (2005): 544.
Max Sherman is president of Sherman Consulting Services Inc. (Warsaw, IN). E-mail him at [email protected].
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like