A new project is exploring the use of simulation during development of treatments for osteoporosis, tuberculosis, multiple sclerosis, coronary stenosis, cerebral aneurysms, mammary carcinoma, and COVID-19 infection.
February 16, 2021
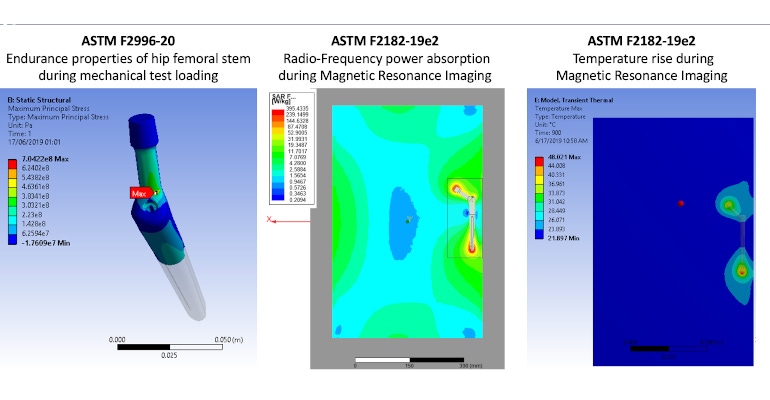
Modeling and simulation are being put to work toward tackling some big healthcare concerns. In January a project coordinated by the University of Bologna and funded by the European Union’s Horizon 2020 kicked off to accelerate adoption of modeling and simulation technologies for medical device and drug development and regulatory assessment, according to project coordinator Marco Viceconti, who serves as professor at Alma Mater Studiorum University of Bologna.
Called In Silico World (ISW), the project takes its name from the online community of practice of the same name, recently launched by the University of Bologna, including experts and all relevant categories of stakeholders of in silico medicine. InSilicoTrials Technologies, among the members of the ISW consortium, offers a platform of models developed to solve complex problems in many different therapeutic areas. The project will develop 11 solutions for In Silico Trials, using state-of-the-art computational technologies to test the safety and/or the efficacy of medical devices, medicinal products, and even advanced therapies such as tissue engineering for regenerative medicine, according to a press release.
“The use of modeling and simulation to develop and de-risk new products is nowadays pervasive, in almost all industrial sectors. However, healthcare is one of the few industrial sectors that has been historically refractory to the use of modeling and simulation in its products development and de-risking,” Luca Emili, CEO and founder of InSilicoTrials Technologies, told MD+DI. “The overall aim of the In Silico World project is to accelerate the uptake of modeling and simulation technologies for the development and regulatory assessment of new medicines and medical devices (known as In Silico Trials), by developing innovative solutions that address the main barriers to their uptake. The results of the ISW project will help to accelerate the adoption of In Silico Trials, increase the trust in these innovative technologies by the main stakeholders (mainly medical industry, regulatory agencies, clinicians, healthcare providers, healthcare payers, policy makers), change the design of regulatory trials to include in silico technologies, and consolidate the regulatory pathways based on modeling and simulation results. The long-term impact will be the reduction of the costs and duration of the development and regulatory assessment of new medical products, while maintaining or improving the level of safety provided by conventional approaches.”
Use of modeling has previously been “scarce” because “the human body is excessively complex to be modeled, and that for most physiological and pathological processes of interest, the causal and quantitative knowledge required to build mechanistic computer models is lacking,” Emili said. “However, research activities have showed that in many cases there is already enough knowledge to build accurate models, and that the resulting complexity could be tamed with the flexibility of modern modeling technologies and the huge computational power now available.”
Emili said that two specific objectives of the ISW project are “to develop better and more advanced validated models for the evaluation of safety and efficacy for both medicinal and medical device products that address specific context of uses, but at the same time enable a completely new class of models of disease with a similar pathogenesis; and to improve scalability and efficiency of In Silico Trials technologies in order to handle big data for large-scale simulations. In the long term, the adoption of models and solutions developed during the ISW project will allow to drastically reduce animal testing and human testing, lower the costs and duration of development and regulatory assessment of new products (and thus their prices), and shorten their time-to-market, while preserving or improving the levels of reliability and safety provided by the traditional pathways.”
The 11 solutions will focus on medical products to treat osteoporosis, tuberculosis, multiple sclerosis, coronary stenosis, cerebral aneurysms, mammary carcinoma, and COVID-19 infection. “The ISW consortium includes 14 partners from 6 European countries, with expertise in several medical applications (neuromusculoskeletal, cardiovascular, neurovascular, transmissible diseases, regenerative medicine and oncology), bioengineering, computer science and cloud infrastructures, commercial exploitation, regulatory science, and legal and ethical aspects of digital technologies,” explained Emili. “ISW project will support the trajectory of 11 In Silico Trials solutions, covered by the broad expertise of the consortium, through their entire product life cycle, encompassing the following enabling phases: development, validation, regulatory approval, optimization, and commercial exploitation. These 11 solutions will target different medical specialties (endocrinology, orthopedics, infectiology, neurology, oncology, cardiology), as well as different diseases (osteoporosis, dynapenia-sarcopenia, osteoarthrosis, tuberculosis, multiple sclerosis, mammary carcinoma, arterial stenosis, cerebral aneurisms), and different types of medical products (medicinal products, medical devices, and Advanced Therapeutic Medicinal Products). As each of these solutions will successfully be developed, the ISW consortium will disseminate towards the community that is forming around the use of In Silico Trials, the availability of a number of resources (technologies, validation data, first in kind regulatory decisions, technical standardization plans, good modeling practices, scalability and efficiency-improving solutions, exploitation business models, etc.) that will permanently lower barriers to adoption for any future development.”
The ISW project officially began in January 2021, Emili reported, but it “can leverage a number of important synergies with other initiatives such as the In Silico World community of practice, VPH Institute, Avicenna Alliance, and numerous international research projects,” he said. “The ISW consortium has already identified six most important barriers to be faced and lowered for a faster uptake of In Silico Trials: lack of advanced models, lack of validation collections, no clear regulatory pathways, poorly informed stakeholders, poor scalability and efficiency, and lack of trained workforce. In parallel, the ISW consortium will work with a large multi-stakeholder advisory board to form a community of practice around In Silico Trials, where academics, industry experts, regulators, clinicians, and patients can develop consensus around Good Modeling Practices. InSilicoTrials Technologies will lead the activities to move the project solutions currently under development toward their commercial exploitation.”
Seventeen legal entities from six different European member states (Italy, Belgium, The Netherlands, Germany, Poland, and Hungary) are participating in the In Silico World consortium. It includes seven universities (Alma Mater Studiorum – University of Bologna; Universiteit van Amsterdam; Eindhoven University of Technology; Università degli Studi di Catania; Katholieke Universiteit Leuven; University of Liège; Budapest University of Technology and Economics), three companies (InSilicoTrials Technologies S.p.A.; Mimesis srl; RS Print NV), four research hospitals (Istituto Ortopedico Rizzoli Bologna; Garibaldi Hospital, Catania; National Institute of Clinical Neurosciences Budapest; Erasmus Medical Centre), an international research centre (Sano Foundation), a non-governmental organisation (VPH Institute), and one standardization body (DIN, Deutsches Institut für Normung e.V.).
About the Author(s)
You May Also Like




