Victor Gura, MD, inventor of the Wearable Artificial Kidney, spoke at MD&M West 2019 about design considerations, obstacles, and goals involved with developing a portable dialysis device.
February 5, 2019
If you've ever known someone who has had to be on dialysis, you know that the quality of life for dialysis patients is not great.
First of all, there is a pill burden.
"My patients need to eat 20 to 30 pills a day," Victor Gura, MD, an internist, nephrologist, and inventor of the Wearable Artificial Kidney (WAK) told MD&M West attendees on Tuesday. "So one was making a joke. He said 'I could put them in a bowl with some dressing and have a salad'. The bottom line is it's a burden and it's also expensive."
In addition to the pill burden, dialysis patients are restricted in the amount of water and other fluids they can drink because if their kidneys are not working they retain water. They also cannot eat salty foods for the same reason.
Patients frequently end up in the hospital, they're often chronically thirsty, they can't eat what they want, and don't sleep well. Many dialysis patients have trouble holding a job because they have to go to dialysis three days a week for four hours at a time so they end up on disability. All of these issues and more take a toll on their mental health, which is why many dialysis patients also suffer from depression.
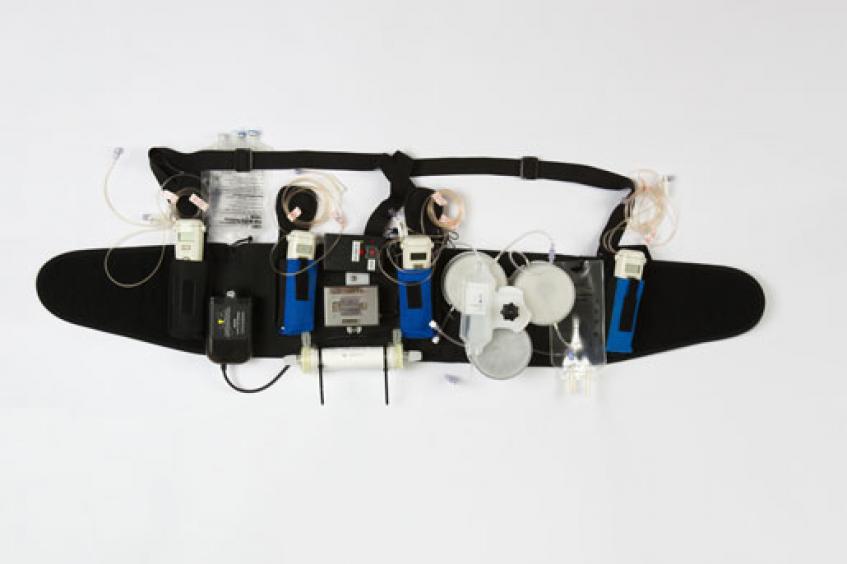
So Gura and his team at Cedars-Sinai Medical Center in Los Angeles set out to take a 300-pound dialysis machine and make it small enough to be worn on a belt.
The Wearable Artificial Kidney is a portable dialysis device designed to enable patients to experience the benefits of daily dialysis while performing their normal, day-to-day routines. The Wearable Artificial Kidney has a unique design that helps eliminate fluid on a regular basis to reduce strain on the kidneys, lungs, and heart while also reducing blood pressure. 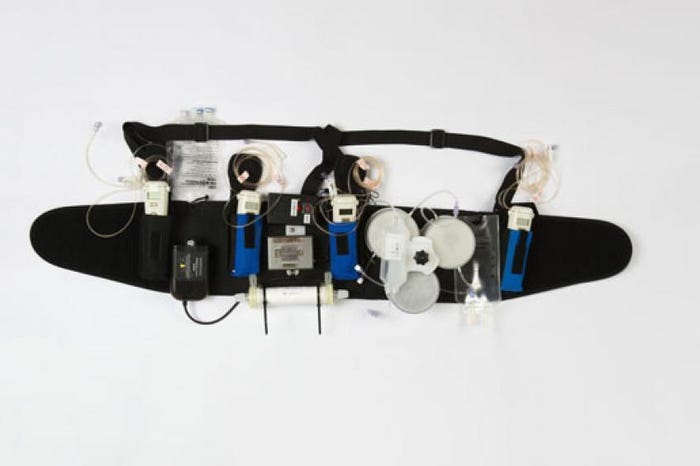
Instead of having to be plugged into an electrical outlet, the Wearable Artificial Kidney is battery operated. The device also requires only 370 CCs of water as opposed to 40 gallons, Gura said. The early iteration of the Wearable Artificial Kidney weighed 11 pounds but Gura said the latest generation of the device weighs only two pounds. The device is connected to the patient via one catheter that is surgically inserted in a 20-minute procedure under a local anesthetic.
There is a risk of clotting and infection with the device, Gura said. "But there are a lot of engineers here who understand risk mitigation, which I had to learn the hard way and we have been very successful in mitigating the risks of both those complications."
Wearable Artificial Kidney: First Clinical Trial
The first clinical trial of the WAK (Wearable Artificial Kidney) ran from October 2014 and April 2015 and included seven patients. They drank water at will, and ate salty, potassium- and phosphorous-rich foods, which are verboten for hemodialysis patients whose blood is usually filtered three times a week at a dialysis center. The Wearable Artificial Kidney filters the patients’ blood continuously, just like a healthy kidney would.
"This guy was on dialysis for many years, and this was our first human patient ever to put on the device," he said, showing a photo of the patient wearing the Wearable Artificial Kidney. "We were all very nervous. After we turned it on nothing happened, the guy feels well, we didn't kill anybody, he walks, he moves, and after a while he says, 'can I go to the toilet?' I said 'okay, but the door is open'. So he's sitting on the toilet having a bowel movement and he says, 'can I have a phone?' I gave him a phone. He calls his wife and says, 'guess what I'm doing while having dialysis?'"
The story got quite a few chuckles from MD&M West attendees but Gura said the sad fact of the matter is traditional dialysis would not allow that man to use the bathroom at all.
"Imagine this guy for 10 years, three times a week in a room with 20 other people confined to a chair for four hours. He cannot have a bowel movement. What if he needs to have one? What if he has a little diarrhea and all the poop is in that chair? It's no fun," he said. "When you put that in a human perspective, wearable devices are important because the quality of life is completely different. It's a different story, it's just not the same."
Having dialysis four hours at a time three days a week falls significantly short of removing water continuously as healthy kidneys do. That's why, Gura said, current dialysis outcomes are less than ideal.
"It doesn't work very well. People are sick, people are miserable. So the only way you can remove the excess water at the same rate that the natural kidneys do is to make it wearable," he said.
Dialysis patients have to take about 12 pills a day to help them get rid of the high phosphates in their blood that comes from food. With the Wearable Artificial Kidney, those pills become obsolete, Gura said, because the device works around the clock just as healthy kidneys do. That alone takes about $5 billion out of the U.S. healthcare system, he said.
So far the Wearable Artificial Kidney has been in development for 18 years, Gura said in response to one attendee's question. And while that might seem fairly fast, given the significant innovation of taking such a therapy that is traditionally done with a huge machine and shrinking it down to a wearable size, from Gura's perspective 18 years has been slower than what he would have liked.
"It took us less time to put a man on the moon since the President said we would," he pointed out. "And believe it or not, the main obstacle has been money."
"We were fortunate enough to win a couple awards from the FDA for innovation and we were fast-tracked, so we do not have a regulatory issue," Gura told MD+DI. "The FDA wanted initially five U.S. clinical trials, but after the first clinical trial we did in Seattle they reduced that to two. So we're two clinical trials away. I think we're about two years from funding the next round to be in the market."
When asked about the next steps in development and whether that might include the use of advanced technologies such as artificial intelligence or new materials.
"Believe it or not, I'm going to be very candid, the main obstacle is money," Gura said. "Having said that, are we looking at new materials? Yes. Are we looking at new polymers? There are many, many things that we're doing but, you know, you're only as good as the money you have."
Gura said he put up the initial funding for the Wearable Artificial Kidney project himself, and then raised money through friends and family and other investors.
"You know, when you enter a business like that, you're also going to disrupt the market, and not very many people want to go up against the big guys. I'll give you an example, 10 to 15 years ago if you wanted to rent a video you went to Blockbuster. Now there's Netflix. Today, in every neighborhood there is one [or more] dialysis units."
In other words, major dialysis companies may not be overly excited about a device that could potentially put them out of business, or at the very least take away some of their market share.
Wearable Artificial Kidney Design Challenges
"We had to overcome many challenges," Gura told MD&M West attendees. "Our main pump, which is our invention, didn't work very well, we had to redesign that. It turned out that an 11-pound device was not something that patients were willing to accept. It was still too heavy. Imagine trying to get on a bus in Jerusalem or get into a plane with that thing."
So people want something less cumbersome and less conspicuous so that they can wear it in public without drawing too much attention to themselves. Early on in the development of the device, the issue of noise came up because people don't want to wear a noisy device that could disrupt their lifestyle, so that was another design challenge that Gura's team had to address.
"At the end of the day you have to please the patients, so we had to come up with something smaller, and we did. We made a list of several tasks that needed to be accomplished by the device. And it turns out that part of the tasks that had to be accomplished could be accomplished with a heavier device over eight hours. So we did a day mode and a night mode. The day mode is very tiny, it does things that need to be done over 24 hours, and the night mode does things that need to be done over eight hours but they are heavier. So we have a day mode and a night mode. At night nobody is walking around with the device, they're in bed. So that allows you to recharge the battery, and at the same time perform certain clinical tasks that can be done over eight hours so you don't have to run around with the heavier device."
About the Author(s)
You May Also Like




