Big medtech companies are all developing devices whose functionality is not hampered when patients undergo magentic resonance imaging (MRI), a common diagnostic tools these days.
May 11, 2016

The numbers tell the story behind what is leading large medtech to develop implantable devices that can work even when patients undergo MRI scans.
Arundhati Parmar
Big medtech companies are all developing devices whose functionality is not hampered when patients undergo magnetic resonance imaging (MRI), a common diagnostic tools these days.
In early May, Medtronic announced the FDA clearance of its MR-Conditional StrataMR valves and shunts that are part of the company's Strata line of Adjustable Valve Systems used in the treatment of patients with hydrocephalus and cerebrospinal fluid (CSF) disorders. In February it announced the availability of an MR-Conditional cardiac resynchronization therapy (CRT) device and the Irish medtech company has a suite of MR-Conditional devices including pacemakers, ICDs, Deep Brain Stimulation systems and spinal cord stimulators allow full-body MRI access to patients.
Stay on top of medtech trends and attend the MD&M East conference at the Jacob Javits Convention Center in New York, June 14-16 |
Other companies are also not lagging too far behind, and in fact Biotronik was the first company to receive CE-Mark for a MR-Conditional CRT-D back in 2012. In late April, Boston Scientific announced FDA approval for its MR-Conditional ImageReady pacemaker, and St. Jude Medical's Assurity pacemaker, similarly safe to use with MRI, is expected to launched in the second half of the year.
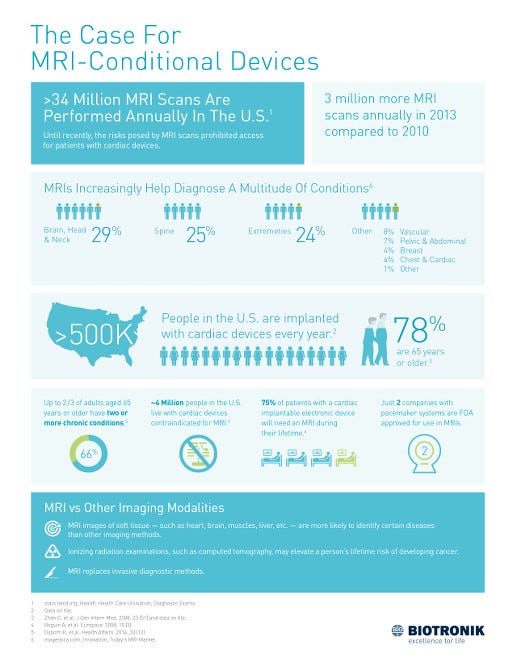
But why are all these companies racing to introduce this functionality into their implantable cardiac and other devices? An infographic created by Biotronik provides some answers. Click image for a larger version
Arundhati Parmar is senior editor at MD+DI. Reach her at [email protected] and on Twitter @aparmarbb
You May Also Like


