The Amsterdam, Netherlands-based company revealed details regarding its upcoming DEFINE GPS global multicenter study. The 3,000 patient trial will assess the outcomes of PCI procedures guided by integrated iFR and interventional X-Ray images. The trial announcement comes on the heels of Philips making a bid to sell its appliances unit so it can focus more on healthcare.
February 21, 2020
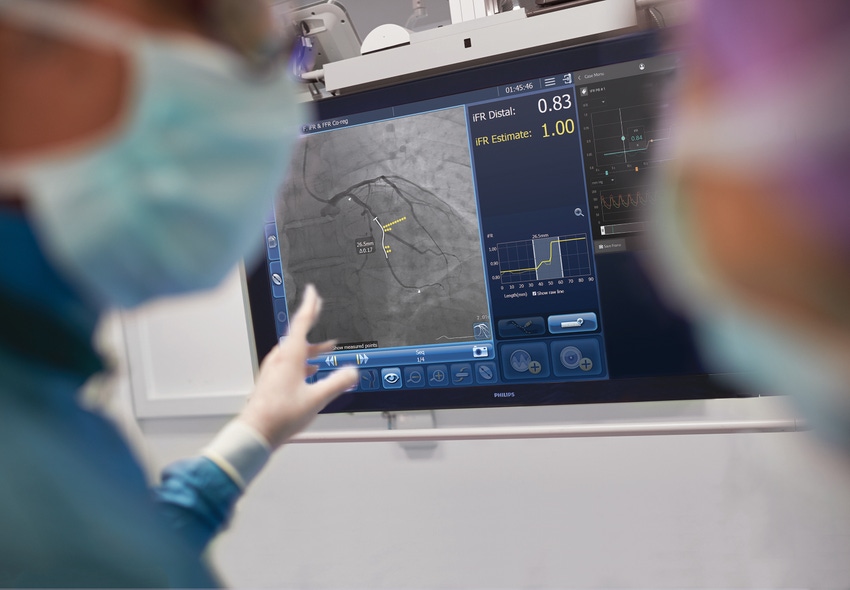
Philips is continuing its investment into percutaneous coronary intervention (PCI) procedures through a new study. The company announced the (Distal Evaluation of Functional performance with Intravascular sensors to assess the Narrowing Effect: Guided Physiologic Stenting) (DEFINE GPS) global multicenter study, which has a goal to assess outcomes of PCI procedures guided by integrated iFR and interventional X-ray images. The announcement of the study comes on the heels of Philips making a bid to shed its appliances unit so it can focus on healthcare.
The primary endpoint is target vessel failure (a composite of cardiac death, target vessel Myocardial Infarction and ischemia-driven target vessel revascularization) or rehospitalization for progressive or unstable ischemia at two years.
About 3,000 patients will be evaluated in 75 centers around the world, said Andrew Tochterman, VP and general manager of coronary at Philips. Enrollment is expected to be completed in the late 2022 timeframe. There will be a one-year-follow-up with results possibly coming in late 2023 or early 2024.
“As medicine evolves there’s increasing pressure on hospitals and physicians to confirm or certify that the patient is going to do well when they leave,” Tochterman told MD+DI. “If we can get more precise and locate where the ischemia-producing lesions are in the vessel, our hypothesis is that, that should produce a higher-quality of life and higher-symptom relief for patients and lead to less downstream readmissions.”
Under the current standard of care, clinicians navigate a balloon catheter and coronary stent to the treatment area using interventional X-ray guidance (coronary angiography). In the study, an iFR pullback measurement, which uses pressure wires to map the pressure profile of the disease distribution along the length of the affected vessel, will be overlaid on the X-ray image to guide treatment. iFR is a next-generation physiologic measurement that uses the same pressure guide wires and equipment as FFR but avoids the administration of hyperemic agents to patients.
“iFR continues to be adopted into clinical practice, with mounting evidence that this innovative technology contributes to improving outcomes, reducing costs and enhancing the patient experience,” Chris Landon, Senior Vice President and General Manager Image-Guided Therapy Devices, Philips said in a release. “This major study will provide a definitive answer to the question of the overall improvement resulting from the use of a functional guidance strategy on patient outcomes and cost.”
The study pays off on the investment Philips made in the PCI space when it acquired Volcano and Spectranetics.
About the Author(s)
You May Also Like




