February 9, 2016
The global in vitro diagnostic test market could exceed $10 billion by 2021, says research and consulting firm GlobalData. And a new hand-held concussions test in the works could give Philips a larger slice of the pie.
Nancy Crotti
|
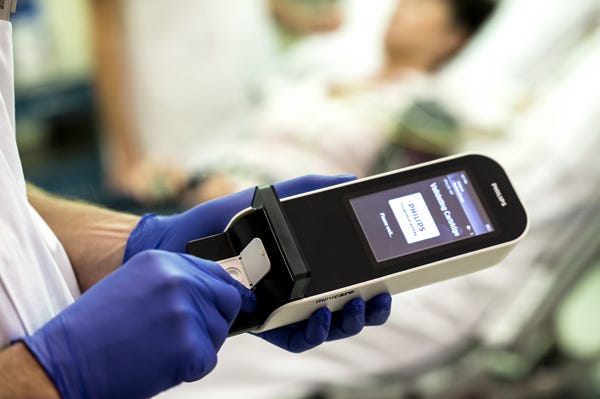
The handheld concussions test under development will be based on Philips' Minicare I-20 system. (Image courtesy of Royal Philips) |
A hand-held blood test to diagnose mild concussions could boost Royal Philips' share of the in-vitro diagnostic testing market, according to an analyst with GlobalData.
Philips is developing the test with with Banyan Biomarkers (San Diego); it is slated to hit the market by 2022. The test uses two brain-specific protein biomarkers that rapidly appear in the blood after a brain injury. Mild and severe traumatic brain injuries (TBIs) can both cause disability, but the milder version (mTBIs) are more difficult to diagnose.
Public attention has been swirling around the effects of TBIs on young athletes, as well as disabling cognitive conditions that professional American football players suffer years after they quit the game. The result is that there is a scramble underway to find devices that help prevent brain injuries.
Jordan Betel, GlobalData's medtech analyst, believes the new device will help reduce the costs of diagnosing mTBIs. The Centers for Disease Control and Prevention conservatively estimates those costs at $17 billion a year in the U.S. alone. The World Health Organization considers them the leading cause of disability in children and young adults worldwide.
"A rapid and objective point-of-care test to evaluate traumatic brain injury will help millions of patients throughout the world," said Jackson Streeter, MD, CEO of Banyan Biomarkers, said in a January news release.
CT scans and MRIs currently used to diagnose TBIs are expensive and time-consuming, and can be inaccurate, according to Betel. "Failure to make a correct diagnosis and timely selection of the appropriate treatment can have serious implications for the patient, and may be associated with the subsequent development of serious, long-term neurological consequences, emphasizing the need for improved diagnosis and acute medical intervention," Betel wrote.
The Philips device uses biosensor technology that "concentrates, separates, and detects target molecules by using magnetic nanoparticles," Betel explained.
The analyst added that Philips plans to offer a range of tests for the new device. As providers adopt such point-of-care devices for clinical settings, this customization should strengthen the company's in- vitro diagnostic test market position, Betel wrote.
Philips has been working hard improve its medtech earnings, which suffered through much of 2015. The Netherlands-based multinational is working with MIT to transform the way brain injuries are monitored. The fully non-invasive calibration-free, portable technology would monitor intracranial pressure (ICP) through a combination of Philips ultrasound technology and MIT physiological modeling.
The company has also made its Lumify smart device ultrasound available to U.S healthcare providers via subscription. Lumify plugs into a tablet computer via its USB port. The interface runs off an app on a smart device.
Nancy Crotti is a contributor to Qmed and MPMN.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like