October 4, 2013
Sterile, nonsterile device packaging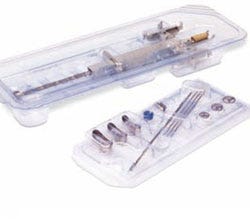 Barger, the medical division of Placon, supplies nonsterile and double-barrier sterile packaging systems for critical applications. The company provides turnkey thin-gauge, rigid plastic packaging solutions for medical devices, applying expertise in the thermoforming of polyethylene terephthalate (PET), glycol-modified PET (PETG), highimpact polystyrene, polypropylene, and high-density polyethylene. Offering thermoformed trays, blisters, clamshells, special protective packaging, mounting cards, lidding, and folding cartons, the service provider performs packaging operations under ISO Class 8 cleanroom conditions.
Barger, the medical division of Placon, supplies nonsterile and double-barrier sterile packaging systems for critical applications. The company provides turnkey thin-gauge, rigid plastic packaging solutions for medical devices, applying expertise in the thermoforming of polyethylene terephthalate (PET), glycol-modified PET (PETG), highimpact polystyrene, polypropylene, and high-density polyethylene. Offering thermoformed trays, blisters, clamshells, special protective packaging, mounting cards, lidding, and folding cartons, the service provider performs packaging operations under ISO Class 8 cleanroom conditions.
Barger
ELKHART, IN
Custom packaging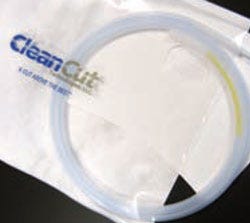 Offering full-range medical device packaging services, CleanCut Technologies specializes in manufacturing compliant device packaging systems in a controlled environment. The company operates ISO Class 7 and Class 8 cleanrooms in its ISO 13485-certified facility and offers full traceability of the raw materials it uses. In order to ensure full performance effectiveness, the service provider develops and designs packaging in consultation with customers. For example, it offers a patented clipless dispenser that protects guidewires and catheters while minimizing the environmental impact of packaging materials.
Offering full-range medical device packaging services, CleanCut Technologies specializes in manufacturing compliant device packaging systems in a controlled environment. The company operates ISO Class 7 and Class 8 cleanrooms in its ISO 13485-certified facility and offers full traceability of the raw materials it uses. In order to ensure full performance effectiveness, the service provider develops and designs packaging in consultation with customers. For example, it offers a patented clipless dispenser that protects guidewires and catheters while minimizing the environmental impact of packaging materials.
CleanCut Technologies
ANAHEIM, CA
NO2 sterilization services Noxilizer Inc. provides medical device manufacturers with contract sterilization services based on nitrogen dioxide (NO2) technology. Specializing in the treatment of drug-device combination products, the company also supplies industrial NO2 sterilizers to customers wishing to sterilize products in-house. Its proprietary room-temperature NO2 sterilization technology compares favorably with traditional sterilization methods using EtO, gamma irradiation, and hydrogen peroxide in terms of safety and the length of processing cycles. The standard cycle lasts 60 to 90 minutes and features immediate release. NO2 sterilization maintains material properties, requires no additional aeration, leaves no cytotoxic residuals, and is compatible with such combination products as prefilled syringes, collagen scaffolds, and bioresorbable implants.
Noxilizer Inc. provides medical device manufacturers with contract sterilization services based on nitrogen dioxide (NO2) technology. Specializing in the treatment of drug-device combination products, the company also supplies industrial NO2 sterilizers to customers wishing to sterilize products in-house. Its proprietary room-temperature NO2 sterilization technology compares favorably with traditional sterilization methods using EtO, gamma irradiation, and hydrogen peroxide in terms of safety and the length of processing cycles. The standard cycle lasts 60 to 90 minutes and features immediate release. NO2 sterilization maintains material properties, requires no additional aeration, leaves no cytotoxic residuals, and is compatible with such combination products as prefilled syringes, collagen scaffolds, and bioresorbable implants.
Noxilizer Inc.
BALTIMORE
Cleanroom packaging, pouch sealing Already equipped with several ISO Class 8 and ISO Class 7 cleanrooms for molding, assembling, and packaging plastic components, Plastics One Inc. has added independent cleanrooms for medical device packaging and medical pouch sealing. These cleanrooms are certified to ISO Class 7 standards for sterile barrier packaging and molding. The service provider works directly with customers worldwide to create advanced package designs. Its ISO 13485-and ISO 9001-certified facility includes an in-house design department using 3-D software, a fully equipped mold-making shop, and current injection molding technology. The company additionally offers a dedicated R&D team, enabling it to provide contract packaging services extending from product concept to creation.
Already equipped with several ISO Class 8 and ISO Class 7 cleanrooms for molding, assembling, and packaging plastic components, Plastics One Inc. has added independent cleanrooms for medical device packaging and medical pouch sealing. These cleanrooms are certified to ISO Class 7 standards for sterile barrier packaging and molding. The service provider works directly with customers worldwide to create advanced package designs. Its ISO 13485-and ISO 9001-certified facility includes an in-house design department using 3-D software, a fully equipped mold-making shop, and current injection molding technology. The company additionally offers a dedicated R&D team, enabling it to provide contract packaging services extending from product concept to creation.
Plastics One Inc.
ROANOKE, VA
Sterile packaging and sterilization services Contract manufacturer Life Science Outsourcing Inc. offers sterile packaging and sterilization services to medical device OEMs. Equipment employed in the company's ISO Class 7 cleanroom area includes a variety of tray and pouch sealers. The service provider offers contract steam sterilization in addition to other popular sterilization methods, such as gamma and E-beam irradiation and exposure to EtO and dry heat. It handles first-time sterilization requests and also conducts periodic revalidation of devices. In addition to providing packaging and sterilization services, the contractor can take responsibility for incoming inspection, cleaning, sterile and nonsterile kitting, product assembly, package testing and validation, returns processing and decontamination, loaner kit processing, repackaging, steam-cycle validation, and fulfillment and distribution.
Contract manufacturer Life Science Outsourcing Inc. offers sterile packaging and sterilization services to medical device OEMs. Equipment employed in the company's ISO Class 7 cleanroom area includes a variety of tray and pouch sealers. The service provider offers contract steam sterilization in addition to other popular sterilization methods, such as gamma and E-beam irradiation and exposure to EtO and dry heat. It handles first-time sterilization requests and also conducts periodic revalidation of devices. In addition to providing packaging and sterilization services, the contractor can take responsibility for incoming inspection, cleaning, sterile and nonsterile kitting, product assembly, package testing and validation, returns processing and decontamination, loaner kit processing, repackaging, steam-cycle validation, and fulfillment and distribution.
Life Science Outsourcing Inc.
BREA, CA
About the Author(s)
You May Also Like


