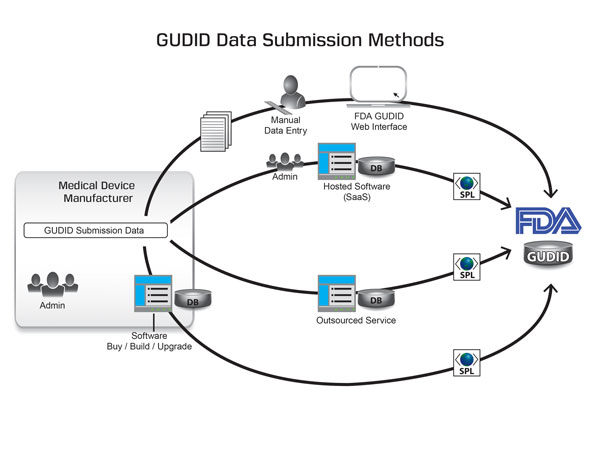
Medical device manufacturers can submit UDI data to FDA using one of four methods: FDA’s GUDID Web interface, custom software, a vendor-provided system, or an outsourced service.
April 16, 2014
(click to enlarge)
A few short months from now, manufacturers of Class III medical devices must comply with FDA’s unique device identification (UDI) rule. Part 1 of this series focused on the need for companies to start planning now for UDI and GUDID compliance. In a conversation with Gary Saner, senior manager of information solutions—life sciences at Horsham, PA–based Reed Tech, Part 2 will get into the nitty-gritty of how to submit data to the Global Unique Device Identification Database (GUDID).
MDDI : UDI and GUDID compliance is upon us. What resources exist for helping companies to make the leap?
Saner: Six months ahead of the first deadline for new federal labeling and data submission requirements, many medical device manufacturers are still deciding how to comply with the new mandate. Fortunately, resources are available to help device manufacturers navigate the requirements of FDA’s final rule on unique device identification.
Published last fall, the new UDI rule is designed to help identify and track medical devices used in the United States by requiring standardized product labels. It also requires that manufacturers collect information on their devices in the publicly available Global Unique Device Identification Database. The new regulations are expected to increase patient safety and healthcare system efficiency.
One of the biggest challenges manufacturers face is collecting, processing, and submitting data to the GUDID. Companies will have to gather substantial amounts of data for each individual product they make. Each product entry will require 55 data values, while FDA’s system will generate an additional seven, for a total of 62 data fields.
Transmitting such vast amounts of data to FDA is a daunting prospect, and time is running out. For example, labelers of Class III medical devices must comply with the UDI label and GUDID submission regulations by September 24, 2014.
The primary components of any UDI compliance solution are a software platform and human resources. The first GUDID submission option involves entering data into FDA’s GUDID Web interface. Manufacturers may submit data to FDA using one of four methods: FDA’s GUDID Web interface, build/buy custom software, a vendor-provided software-as-a-service (SaaS) system, or an outsourced service. Each method requires that labelers first create a free GUDID account, which is currently limited to Class III manufacturers. They can access the site using Microsoft Internet Explorer 9 or 10 or Mozilla Firefox versions 17 to 27. Google Chrome is not currently supported.
MDDI : How does FDA’s GUDID Web interface work?
Saner: FDA’s free GUDID Web interface is an access tool that allows medical device makers to manually enter UDI data directly into the database through a secure online portal. After creating a GUDID account, a user can gain access to the interface tool. While the use of the GUDID Web interface is free, companies will have to shoulder the staff costs and time required to enter data.
The absence of a direct price tag may be tempting to companies whose UDI compliance budget does not afford them as much flexibility as they had hoped. In practice, however, using FDA’s portal to enter UDI information is practical only for companies with a small number of product offerings. Companies with a product catalog of more than 100 devices will have to organize and staff a significant data entry project totaling more than 5500 values. Based on the number of products they offer, even midsize manufacturers will likely have to enter significant quantities of data. And in order to avoid data entry errors, manufacturers will have to budget for a quality check. Companies will also incur ongoing maintenance costs to manually update their records in multiple places should their information change.
MDDI : Could you go into the custom software option?
Saner: Some of the largest companies affected by the new requirements may respond to the looming deadline by developing or purchasing in-house UDI software solutions. Such custom software solutions will require that companies provide both the platform and the staff. Internal systems will allow corporations to bring their existing product catalogs into compliance and to accommodate future needs as their product offerings continue to expand. The investment of time and resources involved in developing an in-house UDI software system, however, could be expensive, making it out of reach for most small and medium-size companies. With the initial compliance deadline of September 24 quickly approaching, it may be too late to begin this type of effort.
MDDI : What about SaaS systems?
Saner: Device manufacturers with a substantial number of products will need to submit larger volumes of data to FDA. Thus, they could benefit from a software program that collects data electronically, converts it into the structured product labeling (SPL) format required by FDA, and submits it electronically to FDA through the electronic submissions gateway (ESG). The advantage of such a system is that it effectively eliminates the costs associated with performing in-house data entry and quality control operations. One such method is the SaaS model, an option that allows companies to ‘rent’ the software solution.
A major difference between the custom software model and the SaaS model is who hosts the software and maintains the system. In the custom software model, the medical device manufacturer is responsible for providing the IT platform, installing and validating it, creating backups, performing maintenance updates, and training users. In the ‘rented’ model, on the other hand, the SaaS vendor provides these services, while the user manages the product data in the SaaS database. SaaS databases can feature search fields or incorporate special fields for entering proprietary information or information required by non-FDA regulatory bodies. Another difference between the two models is cost: Custom software models are generally less cost-effective than SaaS models.
MDDI : What do you have to say about outsourcing the entire process?
Saner: Some companies may choose to outsource their entire GUDID submissions process to a vendor that has the infrastructure, capabilities, and experience to perform this type of work. Using this approach, medical device manufacturers utilize an external provider with a UDI compliance solution and the appropriate experience, such as Reed Tech. This service provider, in turn, processes and submits the data to FDA.
This approach eliminates the need for internal resources to perform GUDID data entry and subsequent maintenance. Vendors can provide accurate data processing services, UDI data validation, experience in SPL and ESG submissions, and the ability to efficiently maintain the data record.
Choosing which GUDID submission approach will work best requires a careful analysis of the time and expertise a company possesses in-house, as well as the costs associated with each of the four options described here.
Bob Michaels is senior technical editor at UBM Canon.
You May Also Like


