February 2, 2016
After seeking to have the lawsuit dismissed, the chief of cardiac surgery at Northwestern University continues to face medical battery charges related to the alleged illegal clinical trial of a cardiology device. A Northwestern teaching hospital and a medical faculty foundation were also named in the suit.
Qmed News
|
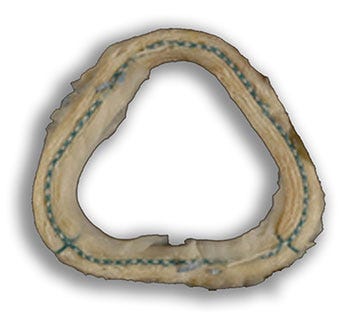
This Model 5100 McCarthy annuloplasty ring was removed from the patient Toni Vlahoulis who was enrolled in the clinical trial. Image courtesy of Toni Vlahoulis. |
Northwestern University's distinguished chief of cardiac surgery, Patrick M. McCarthy, has failed to dismiss a lawsuit alleging that he had implanted an experimental device in roughly 100 patients without informed consent in 2006 and 2007.
The trial against McCarthy, his teaching hospital, and the Northwestern Medical Faculty Foundation, could begin in March in Cook County, IL with Judge William E. Gomolinski presiding.
The Myxo case has also been investigated by Senate Judiciary chairman Chuck Grassley, who from 2008-2014, launched a Senate probe into the possible illegal testing of the Myxo. The Senate Investigation in 2008, revealed documentation of the modifications, and clinical testing of the device using only a patient registry. The modifications were confirmed in Edwards' submissions to the Trademark Office (for the MYXO ETLOGIX trademark) and the Trademark/Service Mark Statement of Use for the DETLOGIX, and then the modifications for the Detlogix submission. In 2014, the Judiciary committee reopened the investigation citing IRB documents were not submitted to the Senator during the initial investigation. Northwestern claims that the Senate approved limited production of documents, but could not confirm this approval with any written statements. A 2014 letter from the Senator questioned whether the university had deliberately neglected to provide relevant documents requested in a prior request from Grassley's office.
The second round of documents submitted by Northwestern University confirmed that, in 2006, the University's institutional review board approved the surgeon's request to treat patients with the Myxo device without informed consent and to retrospectively review the patients' postoperative charts, according to the Medscape.
This was a central allegation in the aforementioned lawsuit by plaintiff Maureen Obermeier, a patient treated with the Myxo device who recently received a judge's ruling that informed consent was necessary to test McCarthy's invention in this case. Obermeier states that she had a heart attack in November 2006 after the Myxo device was implanted.
Ultimately, 667 patients had received the device before FDA ordered McCarthy to stop implanting the device until appropriate regulatory clearance was obtained for the device, according to a 2009 letter from the FDA congressional office to Senator Richard G. Lugar. FDA had argued that the use of the Myxo ring to treat degenerative myxomatous disease of the mitral valve was an indication that would require regulatory clearance.
The FDA Congressional office wrote a patient on July 16, 2009, that the Myxo ETlogix model 5100 would need an investigational device exemption and that soon Senator Richard Durbin from Illinois would be contacting her, which as of today, is yet to happen.
In 2010, Edwards received a warning letter related to the Myxo, which had been rebranded "dETlogix." The letter stated that the device was misbranded because the company had failed to report six serious adverse events related to the device.
McCarthy had argued to FDA that the design changes to the device were minor, and that he therefore thought his actions were justified. McCarthy, however, had given conference presentations in 2007 and wrote journal articles published in 2008 stating that his invention was novel, including in The Journal of Thoracic and Cardiovascular Surgery.
He has long denied any wrongdoing in the matter as do Northwestern University officials.
The Myxo ring, like other controversial devices such as metal-on-metal hip implants, power morcellators, and transvaginal mesh, was ultimately cleared via the 510(k) pathway in 2009. Many of these devices also required safety and efficacy data prior to the 510(k) clearance. The controversy in the 510(k)-cleared devices is now the aftermath of the large number of adverse events reported in the MAUDE database. In the case of the Edwards Lifesciences rings, more than 6000 adverse events listed.
The maker of the device, Edwards Lifesciences, stopped selling the device to the United States market in 2008 because of safety concerns related to the new indication which was not tested in any animal models only human patients, which it termed a "voluntary retrieval" rather than a recall in Edward's 2009 SEC 10-K filings. (It is still marketed in Europe.) Edwards was dismissed from the case by Judge William E. Gomolinski. However, the safety and efficacy study, which now has been ruled, needed informed consent, was the central clinical study necessary for the 510(k) approval as reported by the FDA to Senator Richard Lugar in 2009. A clinical study that failed to report at least one heart attack was exposed in the recent lawsuit in the circuit court of Cook County.
Learn more about cutting-edge medical devices at MD&M West, February 9-11 at the Anaheim Convention Center in Anaheim, CA. |
Brian Buntz is the editor-in-chief of MPMN and Qmed. Email at and follow him on Twitter at @brian_buntz.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like