December 1, 2006
2006 MEDTECH SNAPSHOT
Going global with medical devices must begin with assessing the economics and regulations associated with each country, said Ralph Ives, executive vice president of global strategy and analysis for AdvaMed, at a conference session at BioWest, held August 23–24 in Denver.
Rising expectations of medical technology in nations around the world, he said, will work in device makers' favor. However, these firms need to take into account many factors that hinder access to the market. For example, diagnosis-related groups (DRGs) should help build the market, but many countries are using DRGs to practice foreign reference pricing (i.e., cherry picking the lowest international prices to push down domestic prices).
Device manufacturers, said Ives, must be willing to adopt global techniques. They should make cooperation and collaboration a priority. Device makers have to help governments understand that medical technology is a solution to rather than a cause of problems. To do so, device firms must be willing to share technology and policy data.
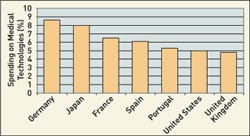
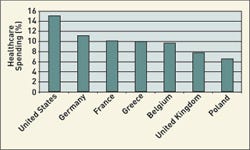
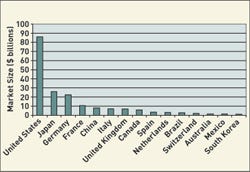
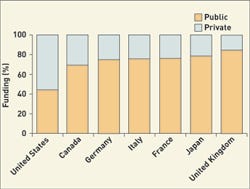
Click images and tables to enlarge:
|
|
Copyright ©2006 Medical Device & Diagnostic Industry
You May Also Like