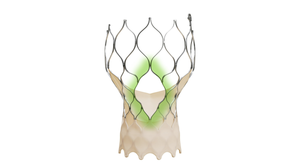
FDA approved Boston Scientific's Farapulse system in January for pulsed field ablation treatment of atrial fibrillation.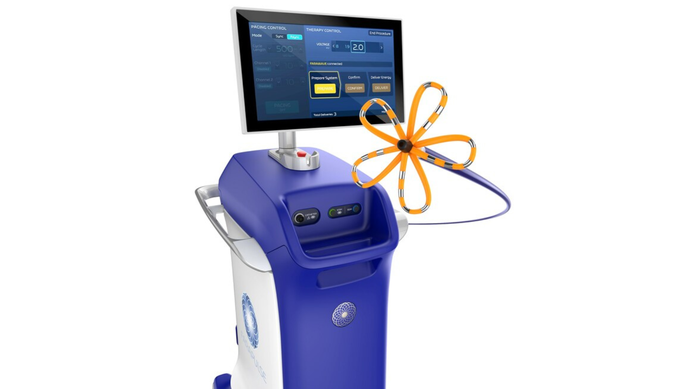
Cardiovascular
Boston Scientific's Farapulse Is a ShowstopperBoston Scientific's Farapulse Is a Showstopper
Electrophysiologists are giving the new pulsed field ablation system a warm welcome.
Sign up for the QMED & MD+DI Daily newsletter.











.png?width=300&auto=webp&quality=80&disable=upscale)














.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)



