There’s a new dynamism at CDRH under director Jeffrey Shuren. While it advances President Obama’s team’s new-broom agenda in the wake of the 510(k) shake-up, it also heeds longstanding industry complaints that seemed to have been placed on the back burner.
July 1, 2010
|
Judging by recent comments, Shuren seems open to sharing some of CDRH’s draft guidance workload with industry. |
One sign of the new listening mode could be seen when Shuren offered a surprise invitation during a Q&A session at FDLI’s annual conference (all the best candor seems to erupt during such unprepped exchanges). He invited industry to submit its own draft guidances if it is frustrated by CDRH’s slowness to produce them. This followed an audience member’s remark that, in view of a clear need for more CDRH guidances to industry and recognizing severe limits on staff resources to produce such documents, consideration might be given to allowing industry to produce draft guidances for approval and publication by the agency. Shuren disarmed everyone by answering: “Write it, but we may or may not accept it.”
It remains to be seen how rapidly industry will pick up on this, but at least it’s an innovative and cooperative approach that doesn’t seem to put CDRH’s independence on the line, which might have been suspected under the prior regime.
Another display of Shuren’s listening-to-you approach came in the announcement that, effective May 1, the center would change the voting procedures for its medical device expert panels. This move may quell the longstanding suspicion that FDA staff manipulate panel deliberations to achieve preconceived outcomes.
As is typical, the official announcement did not acknowledge such suspicion—instead, it innocently cited the increasing number of medical device advisory panel meetings in recent years and the resulting strain on CDRH resources. From now on, though, instead of voting on the approvability of premarket approval applications, including conditions of approval, panels will vote on device safety and effectiveness and risk versus benefit.
The release quoted Shuren as saying the change “is expected to empower the agency to make more-effective decisions that are informed by more clear and focused discussion by panel experts. By making this change in voting procedure, panel members will address key scientific issues during their discussions, which will be reflected in their votes. The change also will allow panel members to address issues related to their area of expertise instead of regulatory issues that may be unfamiliar to them.”
In addition, panels will now vote by ballot rather than by a show of hands. While the votes will be publicly tallied so that panel members will still be identified with their votes, using ballots will allow panelists to cast their votes without the immediate influence of other votes.
Agency staff presentations will continue to include reviews of the staff’s data analysis, but will no longer contain comments on approvability. Reviewers will present the range of scientific opinion in the staff group, rather than a unified consensus analysis of supporting data. The release said that this change will allow more in-depth discussion on safety and effectiveness and risk versus benefit for the device under consideration.
And while continuing its traditional focus on fostering medical device innovation, Shuren told FDLI that CDRH’s first priority is again to implement a “total life cycle approach” to the regulation of medical devices, a focus that seemed to fade under the previous regime. This involves “an effort to fully integrate a comprehensive pre- and postmarket monitoring and compliance program,” he said.
Other steps to improve the quality of premarket data include development of a draft guidance for the design of clinical trials and the development of a unique device identification system, which is critical to tracking medical devices throughout their life cycle. Moreover, he said, CDRH has called on manufacturers for information on “preamendment” devices (i.e., medical devices marketed before they were subject to regulation), and the center is considering what further action, if any, may be needed.
To further promote innovation, CDRH will make available information gained from its vast experience in product regulation to improve product design across all devices—while continuing to shield proprietary information. For example, Shuren said, information readily available from CDRH can lead innovators to come up with improved designs that may reduce radiation exposure or help avoid systemic safety problems known to be associated with like products.
FDA Stops Walgreens Deal on Genetic Test Kit
FDA said in May that it would not allow San Diego-based Pathway Genomics to market an over-the-counter genetic test collection kit at Walgreens drug stores because the test is outside the boundaries of the regulatory safe harbor for laboratory-developed tests. Such tests are regulated by CMS.
In a brief two-paragraph letter to Pathway Genomics, the agency said the firm�’s home-use Genetic Health Report “appears to meet the definition of a device” under section 201(h) of the Federal Food, Drug, and Cosmetic Act. Furthermore, FDA said, there is no FDA clearance or approval on file, and the company must prove its claims that the device is not regulated by FDA but rather by CMS under Clinical Laboratory Improvement Amendments (CLIA).
Simply put—“FDA does not consider distribution of a collection kit that is sold directly to the consumer to be within the boundaries of what is allowed for laboratory-developed tests,” an agency spokesman said.
Based on the letter, Walgreens said it doesn’t want to get into the middle of any beef between the company and the agency. The drug store chain has decided to suspend its decision to allow the kits to be sold at its stores. In a statement, Pathway said, “We respect and understand Walgreens’ decision and we are communicating with FDA about the Pathway Genomics Insight collection kit.”
A Class 1 Recall Designated for Physio-Control Defibrillators
Two months after FDA released all Physio-Control defibrillators from consent decree–mandated restrictions, the products have another problem. FDA has designated as a Class 1 recall a recent voluntary correction of the company’s Lifepak 15 defibrillators manufactured before December 16, 2009.
The company says that it has determined that the affected devices were manufactured with an internal component that could cause an electrical short.The short could then lead to a power loss or the device turning on or off by itself. Loss of power could delay or prevent the delivery of defibrillation therapy, the company says. Physio-Control reports that there have been no adverse events in patients related to this concern.
FDA Issues Warning on Cardiac Science Defibrillators
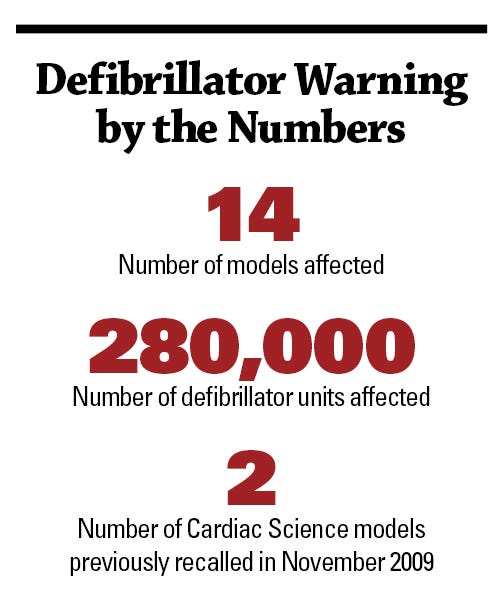 FDA says that faulty components in 14 models of Cardiac Science Corp.’s external defibrillators may cause some 280,000 units to malfunction during attempts to rescue people suffering from sudden cardiac arrest. An agency news release says that the devices are used worldwide in healthcare facilities, public places, and homes.
FDA says that faulty components in 14 models of Cardiac Science Corp.’s external defibrillators may cause some 280,000 units to malfunction during attempts to rescue people suffering from sudden cardiac arrest. An agency news release says that the devices are used worldwide in healthcare facilities, public places, and homes.
The release says that in addition to failing to deliver the needed electrical shocks, the affected devices may have other problems. These problems include interruption of electrocardiography analysis, failure to recognize electrode pads, and interference or background noise that makes the device incapable of accurately analyzing heart rhythm.
FDA is recommending that hospitals, nursing homes, and other high-risk settings obtain alternative external defibrillators and arrange for the repair or replacement of affected Cardiac Science units. For other users, including those who use the device at home or as part of public access programs, FDA recommends using alternative external defibrillators if they are available, and arranging for the repair or replacement of affected models.
If alternative devices are not immediately available, FDA recommends continuing to use the affected devices if needed because they may deliver needed therapy. “The potential benefits of using the available external defibrillators outweigh the risk of not using any of the affected external defibrillators or the risk of device failure,” the news release says.
Last November, Cardiac Science recalled its Powerheart and CardioVive models manufactured between August 2003 and August 2009. But FDA says it has since learned that additional Cardiac Science models—two marketed under the Nihon Koden name and two marketed by GE Healthcare as GE Responder—have similar problems.
The company issued a software update for two of its Powerheart defibrillators in February and plans to issue similar software updates for other affected devices, FDA says. However, the agency also says that its review of the updated software indicates that the software detects only some of the identified defects.
Judge Rejects FDA-Guidant Plea Agreement
A judge has rejected a plea agreement under which Guidant would have paid $296 million and pled guilty to two misdemeanor charges relating to failure to include catastrophic device failure information in reports to FDA. Minnesota federal judge Donovan Frank called on the federal government and the company to consider submitting a modified agreement that would include probation and community service, public education, and charitable activities for the company, and also would send a portion of forfeited funds to Medicare.
|
Judge Frank says he rejected the plea agreement based on several of its provisions, which “are not in the best interests of justice.” |
Despite pleas to Frank that he reject the agreement because it contained no restitution for victims who had problems with Guidant’s Prizm and Renewal implantable cardioverter-defibrillator devices, Frank ruled that there were no victims who had been directly and proximately harmed by the specific misdemeanors to which the company was prepared to plead guilty. Frank also said that his rejection of the plea agreement should not raise hope that alleged victims would receive restitution in a revised agreement.
In his order, Frank reminded Guidant that it could withdraw its guilty plea since the plea agreement had been rejected, but cautioned that such a move could lead to a harsher penalty being imposed by the court. Rejecting that offer, Guidant said it would work with the Department of Justice to develop a modified agreement that would be acceptable to the court, the federal government, and the company.
Frank said he rejected the agreement because two of its provisions
are not in the best interests of justice and do not serve the public’s interests because they do not adequately address Guidant’s history and the criminal conduct at issue. The court respectfully disagrees with the government’s view that probation would be a waste of the taxpayers’ money, especially given that Guidant could be required, as a condition of probation, to reimburse the government for any costs associated with its probation. The court also disagrees with the government’s position that Guidant’s current corporate structure renders any probation meaningless, especially given the fact that Boston Scientific itself recently entered into a $22 million settlement and corporate integrity agreement based on certain pre-acquisition actions by Guidant.
Guidant Corp. became Guidant LLC less than two weeks before the government filed the (criminal) information, according to Frank, who said that “the interests of justice are not served by allowing a company to avoid probation simply by changing its corporate form.” At a minimum, Frank advocates Guidant and Boston Scientific being placed on probation—“regardless of the fact that Boston Scientific acquired Guidant after the events in question.”
As a condition of probation, Guidant, through Boston Scientific, could be ordered to perform community service designed to repair the harm caused by its offenses. The court said that this could help build the public’s confidence in the FDA regulation process, medical device manufacturers’ quality control efforts, and the cardiac healthcare industry in general.
As part of the plea, the judge noted, Guidant had agreed to forfeit $42 million for violations specifically relating to the Renewal device. He said he believed that the interests of justice would be served “only if clear guidelines were established by the government that directed individuals as to how they can petition for the remission of any forfeited funds in this case. The government would also need to have a contact person to answer individuals’ questions concerning this process.” He added, however, that he expects that any forfeiture remission process would determine, as he had, that legally there are no victims of the violation to which the company would plead guilty.
Frank said that given that about 15,000 of the devices at issue went to Medicare recipients and that Medicare likely incurred significant expenses due to the violations, it would make sense for a plea agreement to provide that some portion of the forfeited funds go to Medicare.
AdvaMed and Eucomed Extend Reach of Ethics Code
AdvaMed and the European Medical Technology Industry Association (Eucomed) have approved a joint statement on ethical interactions between medical technology companies and healthcare professionals. The two groups say that although individual industry codes of ethics and business practices have been in place for several years, healthcare and medical technology innovation increasingly extends beyond national and cultural borders.
Under the agreement, the two associations pledge to work together to
? Promote ethical interactions among companies and healthcare providers by encouraging companies to adopt compliance programs and policies consistent with the AdvaMed and Eucomed codes.
? Provide guidance to the medical technology industry at large on ethical business conduct relating to companies’ interactions with healthcare providers.
? Support education and compliance of companies with all applicable laws, regulations, or professional codes (including national association codes) that may impose more stringent requirements relating to companies’ interactions with healthcare providers.
? Work together to advance ethical collaboration consistent with the AdvaMed and Eucomed codes globally through regular communication, joint policies (where appropriate), joint activities, and other appropriate collaborations.
Former CDRH Chief Blasts LASIK Trial as Unethical
Former CDRH chief of ophthalmic devices Morris Waxler, who was in charge of evaluating LASIK devices approved between 1996 and 2000, told the American Society of Cataract and Refractive Surgeons (ASCRS) in May that its proposed Phase 2 keratectasia trial on LASIK effects is unethical. “I will do everything I can to block approval of such a study,” he said. Waxler, who has recently said the agency’s approval of LASIK devices was a mistake, defined keratectasia as “a rare but serious complication of laser vision correction of any refractive errors.”
Waxler’s criticism came in a three-page letter to ASCRS president Doyle Stulting, in which he said keratectasia affects at least 1% of LASIK patients. He also cited a high probability of various other permanent vision and ocular health problems. “LASIK eye surgery complications are already a major public health problem. Hundreds of thousands of eyes are permanently injured each year for a 60% chance of a couple of years free from glasses and contact lenses, and a 40% chance of little or no freedom from spectacles or contact lenses at all. Fifteen to 30% of LASIK patients suffer from eye pain, glare, halos, dry eyes, night vision, and other problems, even if their visual acuity is normal.”
Waxler told Stulting that FDA is “complicit” with LASIK manufacturers, ASCRS, and others in trivializing the seriousness of multiple permanent vision complications. He asked for Stulting’s cooperation in helping to eliminate unnecessary LASIK through more transparency about the short-term “wow” effects versus the reality of permanent eye injury and achieving “clear and complete informed consent documents comparing percentage risks and benefits of LASIK versus glasses or contact lenses.”
Waxler said he planned to publish an open letter to FDA commissioner Margaret Hamburg in major U.S. newspapers this summer, urging specific actions by the agency to “stem the tide of unnecessary LASIK eye injuries.”
Storz Medical AG Cited for Unapproved Devices
A recent CDRH warning letter says that Storz Medical AG, based in Tägerwilen, Switzerland, has been marketing its D-Actor 50 and D-Actor 200 devices in the United States without marketing clearance or approval. The letter said the company does not have premarket approval in effect for the D-Actor 50. The D-Actor 200 was given 510(k) clearance to relieve minor muscle aches and pains and for the temporary increase in local blood circulation.
However, the letter said, the company Web site states that with the devices “tissue is purified” and “the interaction between the central and peripheral nervous system is improved.” CDRH said these statements are a major change or modification in the device’s intended use, which requires a new premarket notification.
Storz was told to immediately stop marketing the two devices for unapproved uses and to respond with a listing of specific steps taken to correct the violations and prevent them from recurring. CDRH also requested documentation of actions taken and a timetable for completion.
About the Author(s)
You May Also Like




