Color-coded cannula systems must be made of material that offers clarity and remains color-stable after sterilization. Image courtesy of EASTMAN CHEMICAL CO.
Clear medical devices are often essential to effective patient care. The transparency of plastic materials in applications such as cannulae, needles, and fluid bags allow healthcare professionals to visually inspect contents, make observations for foreign objects, and monitor for unexpected events, such as bleeding or the appearance of an infection. In addition, clear, colorless, and brightly tinted plastics are associated with a clean and sterile device, which also aids in patient comfort. Recently, more device applications have taken advantage of color-coded clear plastics to help clinicians identify devices.
To ensure that a device reaches the healthcare setting with its intended color and clarity, manufacturers must understand available sterilization methods and the effect each may have on the color of the polymer used to develop the device.
Importance of Color and Clarity
Healthcare practitioners commonly rely on color-coded medical devices for split-second identification. During critical medical emergencies and procedures, practitioners must be able to quickly, efficiently, and correctly identify and select medical devices based on color, which can indicate the size, type, and function of the device. Such easy identification and quick execution can help reduce medical errors and save time. For example, emergency room nurses often report that color coding helps them find the correct devices quickly, thus reducing the risk of an error. Color-coded devices help provide timely and lifesaving care because healthcare professionals don’t have to question whether they have the right equipment. Color coding also helps surgeons distinguish between different devices and tools.
For example, Smith & Nephew Endoscopy, a manufacturer of arthroscopic devices, developed its Clear-Trac Complete cannula system in nine color-coded sizes. During arthroscopic procedures, cannulae provide sterile pathways to the joint that surgeons will treat. The Clear-Trac Complete system, made with clear and color-stable Eastar copolyester, enables surgeons to identify separate cannulae during surgery and find the best fit based on the size of the patient, the size of the joint being treated, and the thickness of the muscles around the joint. The clarity of the material provides surgeons with an unobstructed view of the instruments and the suture inside them, as well as the bone and soft tissue that surround the surgical site.
In addition to color coding, medical teams often need high-clarity, colorless materials for devices. Polymer clarity provides healthcare practitioners with an unobstructed view to detect foreign substances, bubbles, clot formation, and fluid levels. Recognizing potential issues immediately can prevent significant problems, such as an embolism or insertion site infection, from developing.
Glasslike clarity is also closely associated with cleanliness. If a medical device is not clear, or if it is slightly discolored or its color has shifted as a result of sterilization, clinicians as well as patients may question its sterility and safety. Some nurses report that a dark or discolored device will be thrown away and not used because such discoloration makes it appear to be old and outdated. Clear, colorless devices allow easy monitoring of fluid levels, inspection of a IV site to determine whether an infection is present, and monitoring of fluid colors to determine whether blood is present in a drainage line, for example.
|
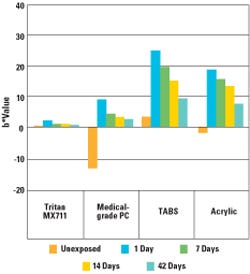
Figure 1. This chart shows b* color measurements of molded resins before and after exposure to 50 kGy of gamma radiation. |
Medical device manufacturers must take color shifting during sterilization into account to ensure that devices maintain clarity and intended color quality. With varying material options available, manufacturers and processors must understand the effect of sterilization on polymer properties. In addition to improving the color and clarity of high-performance materials, material suppliers must continue innovating to develop more-reliable material options that minimize the trade-offs of color stability, toughness, chemical resistance, and processability associated with many materials available today.
How Sterilization Affects Materials
The objective of sterilization is to reduce the bioburden to zero pathogens while minimizing any change to the physical and optical properties of the final part. Autoclave, ethylene oxide (EtO), gas plasma, and exposure to gamma or E-beam radiation are common sterilization methods that have varying effects on color shifts of materials used for plastic components or devices. Selecting the preferred technique for any medical device is critical and must include a thorough evaluation of the interactions between the materials and the sterilization method, especially when clarity and accurate color of plastic components or devices are crucial.
Autoclave Sterilization. An autoclave uses high temperatures and humidity to kill microorganisms. These harsh conditions can cause most common polymeric materials to warp and distort, rendering them useless. In addition, the high temperature and humidity can break down the molecular weight of some polymers through hydrolysis. Polymers that have undergone significant hydrolysis are more susceptible to breakage because their toughness is compromised due to the lower molecular weight. Autoclave sterilization techniques do not typically cause significant color shifts of polymeric materials.
EtO Sterilization. EtO relies on the toxicity of the gas to kill microorganisms. Using EtO to sterilize medical devices can be a relatively slow, three-step process:
? Preconditioning—exposing the packaged devices to elevated temperatures and humidity.
?Sterilization—introducing EtO into the relatively hot, humid chamber (maximum temperature between 60° and 80°C).
?Aeration��—degassing the device and package to remove the toxic EtO before shipping,
Like autoclave sterilization, EtO sterilization also does not typically cause significant color shifts. It can be the most economical method for low-volume devices and parts that are heat sensitive. This method is a good choice for plastics that may undergo physical property degradation when expososed to radiation.
Gas Plasma Sterilization. Hydrogen peroxide gas plasma sterilization provides safe, nontoxic, dry, and low-temperature sterilization in about one hour. The plasma environment generates an ionic and free radical rich environment that reacts with microbes, killing them or otherwise rendering them harmless. Water and oxygen are the primary by-products of this sterilization method, so there is no need for aeration, as required for EtO. One of the disadvantages of gas plasma is that the reactive sterilizing environment may not penetrate well, especially in long channels or devices. Gas plasma sterilization methods typically also cause no significant color shifts in polymeric materials.
Radiation Sterilization. Exposure to gamma or E-beam radiation is a very quick, highly productive device sterilization method. It is exceptionally useful for high-volume applications with consistent pallets and packaging, because it can take advantage of high throughput. The major disadvantage of gamma radiation sterilization is that a cobalt 60 radioactive source is necessary, which requires special handling and extra costs. Exposure to E-beam radiation is a sterilization technique without the difficulties associated with a radioactive source, but it has limitations in penetrating high-density materials such as metals.
|
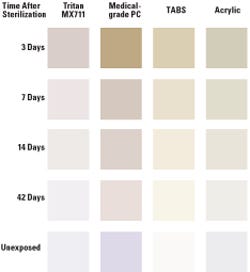
Figure 2. Photographs of molded resins before and after exposure to 50 kGy of gamma radiation. |
Gamma and E-beam radiation easily penetrate both packages and devices to kill or disrupt replication of biological materials by exciting molecules to high-energy states. These methods lead to ionization, bond breaking, cross-linking, or chain scission. Unfortunately, these radiation methods do not discriminate between microorganisms and the polymeric materials used in the device or packaging. The radiation also excites the polymer’s molecules to high-energy states. The mechanisms to dissipate the energy include bond breaking (lowering the molecular weight of the polymer, eventually leading to embrittlement), cross-linking (also eventually embrittling a polymer), recombination (with essentially no effect on the mechanical properties of a material), and rearrangements that can result in the formation of color bodies (which may fade slightly over time). Each of these energy-dissipating mechanisms occurs at different rates depending on the polymer, leaving some polymers extremely discolored upon exposure to radiation and others essentially untouched.
Polymeric Materials and Radiation Exposure
If retention of highly accurate color in a device is critical, EtO, gas plasma, or autoclave sterilization methods typically have little or no effect on the color shift and color variability of a device.
If gamma or E-beam radiation is preferred for cost, speed, low temperature processing, or other reasons, it is important to understand how to minimize the effect on optical properties through proper material choice. It is critical that medical device manufacturers are aware and educated on the effects of sterilization, which can vary depending on material differences and the dose and source of radiation.
One of the most critical material effects of sterilization is color shifting, particularly because color coding and clarity play such vital roles in the function and reliability of medical devices. Polymers exposed to radiation often shift in color to yellow. For healthcare practitioners, any discoloration or property degradation is a cause for concern. Medical device manufacturers and processors must make educated material choices and work closely with material suppliers to select polymers that offer minimal color shift after sterilization.
Measuring Color Shifting
The effects of color and optical clarity on a material vary depending on the type and dose of radiation, as well as the length of time elapsed after irradiation. A materials manufacturer conducted a study to identify the effect of gamma and E-beam irradiation on several polymers, including medical-grade copolyester, gamma stable polycarbonate (PC), transparent acrylonitrile butadiene styrene (TABS), and acrylic (PMMA).
|
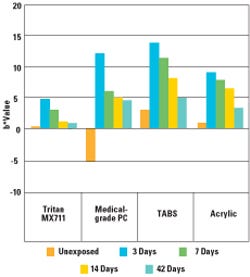
Figure 3. This chart shows b* color measurements of molded resins before and after exposure to 50 kGy of E-beam radiation. |
In the study, plaques of each material were injection molded under conditions typically used to process the polymers. Next, the initial color of the plaques was measured using a HunterLab UltraScan Sphere 8000 and reported using the CIE (International Commission on Illumination) b* color scale. In the CIE b* color scale, increasing positive values indicate increasing yellow color, while negative values indicate blue color. Following initial color measurement, the plaques made from each polymer were exposed to 50 kGy of gamma radiation to simulate a typical medical device validation test. Separate plaques made with each polymer were also exposed to 50 kGy of E-beam radiation. Following exposure to the radiation, the plaque color was remeasured with the HunterLab color method described above at various intervals with the final measurement at 42 days to monitor the initial color shift and decay over time. Photographs of the plaques were taken before exposure and at 42 days.
The results demonstrated that gamma and E-beam irradiation can affect the color of various polymer materials differently. After 50 kGy of gamma radiation, each of the materials showed a positive shift to higher b* values (see Figure 1), indicating yellowing. The Tritan MX711 copolyester showed the least amount of initial yellowing with a shift of only about two b* units. The acrylic, TABS, and PC materials all shifted more than 20 b* units when measured three days after initial exposure to the gamma radiation. Over time, the yellow color faded in each of the test samples, resulting in a final b* value within 0.2 units of the initial color for the Tritan MX711 sample after 42 days. The final color for the TABS and acrylic samples faded to within approximately six and nine units of initial color, while the PC sample faded the least, with a final color shift of 17 units. Photos of the plaques before and 42 days after exposure to gamma radiation are shown in Figure 2.
Similar results were observed after the materials were exposed to E-beam radiation as shown in Figure 3. Tritan MX711 had the lowest yellow shift of any of the materials with a measured b* shift of 4.3 units. The TABS and acrylic shifted approximately 11 and 8 b* units respectively, while the PC shifted the most, with a measured change of 17 b* units. Again, the color faded back toward the initial color over time, with the Tritan MX711 sample coming to within 0.5 b* units. The TABS and acrylic samples final shift was slightly less than 3 b* units, while the final shift in color for the PC sample was more than 9 b* units. Figure 4 shows the molded resins before and after exposure to E-beam radiation.
|
Figure 4. Photographs of molded resins before and after exposure to 50 kGy of E-beam radiation. |
Conclusion
The proper choice of materials that are compatible with the desired sterilization methods can result in optimum functionality and perceived quality of medical devices. Gamma and E-beam irradiation sterilization methods can cause a significant color shift with some materials, such as TABS, acrylic and PC. Gamma irradiation was found to cause more of a color shift than E-beam irradiation in the materials studied. Tritan copolyester MX711 showed the least amount of color shift of all the materials studied, while gamma stable PC was found to have the greatest overall shift in color.
In summary, medical devices often depend on clear and tinted materials for functional and aesthetic reasons. To optimize retention of the desired color of clear or tinted devices, manufacturers need to be aware of the effect of sterilization methods on shifts in color of their devices. Plasma, EtO, and autoclave sterilization methods have minimal effect on the color of typical clear plastics used, but radiation methods can have a big effect. If radiation methods are used for sterilization, copolyesters are shown to have the lowest color shift of any of the materials examined in this study.
Scott A. Hanson, PhD, is the global industry leader of the medical segment at Eastman Chemical Co. (Kingsport, TN). Rachel Turner is manager of marketing insight and strategy at Eastman.
About the Author(s)
You May Also Like