Device makers don’t have to take negative coverage decisions lying down. There are several options to convince payers of the medical necessity of a device.
REIMBURSEMENT
|
Brandel |
One of the most feared phrases in the medical device industry is “the use of (your product) is considered investigational/not medically necessary.” A decision by a local Medicare carrier or a private payer not to cover a new device can bring sales to a standstill. Even if a device maker has done everything right, the possibility always exists that payers will make a determination that the device is not going to be covered. This is most likely to occur if the clinical literature is not airtight or if the device maker does not make a strong economic argument about how the device will lead to savings in overall utilization or expenditures. Short of a significant breakthrough in terms of outcomes and quality of care, payers are looking for a technology that represents a more cost-effective alternative to what is currently available.
|
Grenell |
A negative coverage decision, however, is not necessarily the end of the road. Commercial payers and Medicare both have a variety of mechanisms in place to appeal such a decision, and a number of steps can be taken to modify the decision. As with the initial coverage decision process, the appeals process differs by payer.
|
Lovett |
The Commercial Payers
What should a company do if it finds its products facing a negative coverage decision from a commercial payer? Although payers are under no obligation to follow each other in terms of medical policy, a snowball effect often can occur. Therefore, one of the most important things to keep in mind is that device makers need to react quickly. They shouldn't assume that there is no recourse, nor should they hope that another payer would make a different determination.
The first step is to identify the specific reasons cited for the decision. If at all possible, try to speak with the medical director of the commercial payer to determine specific concerns and elicit opinions on what would be needed to reverse the decision. The most common problem is that the clinical literature does not substantiate the efficacy of the technology. Although the specific process may differ from payer to payer, clinical data are the basis for a large part of the decision-making process. The carrier Wellpoint (formerly Anthem Inc.) has a medical policy that can be used as a template for the specific issues a policymaker considers. It measures the medical necessity of a new technology based on several key issues, as follows:
Does the device have final approval from the appropriate regulatory body?
Is it supported by scientific evidence that allows conclusions regarding the effect of the technology on health outcomes?
Will the device improve net health outcomes?
Does the device compare beneficially with established alternatives?
Has it shown outcomes and improvements that are attainable outside a controlled setting (i.e., in practice)?
Is it viewed as a standard of care in the community with supporting documentation?
Generally speaking, all of these conditions must be satisfied for a technology to be considered medically necessary.
|
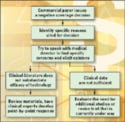
Figure 1. (click to enlarge) This flow charts identifies the steps to take when a negative coverage decision is received from a commercial payer. |
If literature is the reason cited for a negative determination, the device firm should review the materials and have clinical experts develop a point-by-point response. In addition, determine whether there is any positive literature that was not reviewed by the payer that might help the case. It is also important to keep in mind that the medical director of the carrier may not have any direct clinical experience in the specialty in question. Therefore, all the relevant information must be submitted in a manner that can be easily understood by a generalist. Lastly, given the multitude of issues that find their way onto the medical director's desk, don't assume that the carrier will have the time to follow up on references or other materials that haven't been submitted directly to the firm for review. Send everything that can help present the best possible case for coverage.
If, after a careful review of the existing literature, it is determined that the available data are not sufficient, it may be time to evaluate the need for additional studies. Alternatively, a clinical trial that is currently under way can be revised. The new data collected must establish that the procedure or device can either substitute for costlier alternatives or significantly improve outcomes. The additional expense for an expanded clinical trial might be justified if the alternative is to market the device without coverage for the foreseeable future.
Appeals for Commercial Payers
Private payers do not generally have a formal reconsideration process for challenging a coverage decision. It is up to the manufacturer to try to work with each payer and to present the evidence to support the device.
Coverage is defined by the benefit plan that serves as the contractual agreement between the payer and the employer group (or individual). In the case of an insured plan where the payer agrees to provide specified services in return for a monthly premium, the payer has an incentive to limit coverage if there is any likelihood that the new technology will increase costs.
Employer groups can help influence a payer with a convincing argument on a technology's effect on outcomes or quality. Because payers also rely on determinations made by outside technology assessment companies, it is important to heighten the awareness of these companies to new technology and the literature that supports its efficacy.
Private payers do, however, have an appeals process for patients who have had an adverse determination such as a denial on a preauthorization request. Once the internal appeals process has been exhausted, most states now have laws requiring an external appeals process. The external appeals process is generally handled by an inde- pendent review organization that does not have any financial or other interest in either side. When reviewing a case regarding experimental or investigational treatment, the organization and its reviewers consider all information pertinent to the case. That includes existing medical research and peer-reviewed literature. An affirmative decision for the patient does not necessarily mean that the coverage policy will be revised; there may be circumstances unique to that case. It does, however, begin to build the case that can be used in arguing for coverage as an overall payer policy.
Medicare—National Coverage Determinations
A national coverage determination (NCD) is a policy statement that grants, limits, or excludes Medicare coverage for a particular medical item or service. An NCD can be requested by any party, including beneficiaries, providers, and manufacturers. It can also be initiated internally by CMS. Once an NCD is issued, however, it is binding on the carriers. The only opportunity for a manufacturer to overturn an existing NCD that limits or excludes coverage is to submit a reconsideration request. The reconsideration request will only be considered it if is accompanied by additional medical or scientific data that were not part of the initial review or if the requester asserts that the conclusions made as part of the initial review process misinterpreted the data. The reconsideration process generally takes 90 days and the existing NCD remains in effect during this period. Because of the difficulty of overturning an NCD, most manufacturers are reluctant to request a national policy unless the scientific evidence is clear and convincing.
Medicare beneficiaries who are aggrieved parties (i.e., a beneficiary who is in need of the item or service but was denied coverage) have an additional option for appealing an NCD. They can appeal to the Departmental Appeals Board by filing a formal written complaint. Within 90 days of completing its review, the Departmental Appeals Board must issue its decision or indicate the approximate date of a pending decision. Once all administrative remedies have been exhausted, a beneficiary can seek judicial review in a federal district court. The NCD appeals process for a beneficiary is distinct from the appeal rights related to the adjudication of Medicare claims. A claims appeal allows a beneficiary to submit any information relating to his or her claim but can only affect individual situations. An appeal related to a national or local coverage determination can amend the policy itself and applies to all affected beneficiaries.
Medicare—Local Coverage Determinations
If Medicare has not issued an NCD, the local carriers have the ability to issue coverage decisions on a local basis. Note that as a result of the Medicare Modernization Act of 2003, Medicare intermediaries and carriers are now being replaced with new entities called Medicare administrative contractors, or MACs. By 2011, there will be a total of 23 MACs throughout the country, 15 of which will cover most of Medicare Part A and Part B services. The MACs will assume the responsibility for the local coverage determinations (LCDs).
Each carrier follows specific rules and regulations in issuing LCDs and in considering appeals. Issuing a draft LCD for a device or technology is generally the first step a carrier takes. If the draft indicates that the carrier considers the technology investigational or is planning to cover it in only limited circumstances, the manufacturer should take the opportunity to submit further evidence during the comment period. Just as is the case with the private payers, local carriers base their decisions on published authoritative evidence and general acceptance by the medical community (standards of practice). Such general acceptance evidence is based on the following:
Scientific data or research studies published in peer-reviewed medical journals.
A consensus of expert medical opinion (i.e., recognized authorities in the field).
Medical opinions derived from consultations with medical associations or other healthcare experts.
The strength of the argument is directly related to the strength of the evidence presented in the clinical literature. The comment period for a draft LCD is a minimum of 45 days, which gives a manufacturer time to develop its response and enlist the support of the provider community.
Medicare—The Reconsideration Process
If a final LCD is still not acceptable, there is a reconsideration process that enables interested parties to request a change. The request can be submitted at any time by beneficiaries residing or receiving care in the carrier's jurisdiction, providers doing business in the jurisdiction, or any interested party doing business in the jurisdiction (e.g., the manufacturer). The reconsideration process is relatively straightforward and is outlined on every carrier's Web site. Reconsideration requests can only be submitted for final LCDs and are held to the same standard of evidence as the original LCD. Within 30 days of receiving the request, the carrier determines whether the request is valid (i.e., whether it relates to a final LCD, whether it conflicts with an existing NCD, or whether the requestor meets the evidence requirements). If valid, the carrier then has up to 90 days to make a decision on whether a revision is warranted. Because final LCDs are generally required to have a 45-day notice period before the policy goes into effect, the reconsideration request should be filed as soon as possible to avoid gaps in coverage.
The only other formal appeals process for a Medicare local carrier determination is an LCD challenge, which can be filed by a Medicare beneficiary (or the estate of a Medicare beneficiary) who is in need of a service that would be denied by the LCD. An administrative law judge, who will make a decision after reviewing the evidence, reviews the challenge. If the judge rules in favor of the beneficiary, it may trigger a change in the LCD. However, the local carrier may appeal such a decision to a Departmental Appeals Board. Decisions rendered by the Departmental Appeals Board are then subject to further judicial review.
Strength in Numbers
Chances are, if you have received a negative coverage determination from a private payer or from Medicare, there are other manufacturers who have been similarly affected. In this case, consider the possibility of working with competitors to convince the approval bodies of the benefits of the technology. Most manufacturers belong to associations that have committees assigned to focus on issues related to reimbursement and coverage. There may be an opportunity to develop a strategy to overturn a negative decision. Activities might involve initiating a letter-writing campaign from the combined customer base in the community, developing model letters to be used by the providers, or requesting a meeting or conference call with the payer. Of course, legal counsel should review any strategy involving competitors to ensure that there are no antitrust or other issues that might prevent the companies from working together.
In addition to other manufacturers, enlisting the support of patient groups, disease-specific foundations, and employers can be highly effective. Although it may be relatively easy to resist one or two manufacturers, payers will have a much more difficult time ignoring the appeals of their constituent groups.
The Common Denominator
The common denominator in all the appeals processes, whether directed at Medicare or a private payer, is the need for solid clinical literature that demonstrates the efficacy of the procedure or the device. The device must be held to the same standard of evidence that was used in making the initial determination. It is therefore essential to focus on presenting a clear and convincing argument, backed by clinical data that are easily understood by the individuals that make coverage determinations. The most important component of a manufacturer's sales and marketing strategy should be to develop a study design that answers critical questions from the payers' perspective. It is well worth the up-front investment. Without supporting data, what you spend in time and resources after the fact, combined with the missed sales opportunities, will surely outweigh the initial cost of a more comprehensive study design.
Debbie Brandel is a principal with Preferred Health Strategies (PHS; Rye Brook, NY) and can be contacted at [email protected]. Barbara Grenell is president of PHS and can be reached at [email protected]. John F.X. Lovett is a principal with PHS and can be e-mailed at [email protected].
Reference
1. Medical Policy Formation, effective date 12/07/06 [online] (Indianapolis, IN: WellPoint Inc. [cited 31 January 2007]); available from Internet: www.anthem.com/medicalpolicies/noapplication/f3/s1/t0/pw_a038688.pdf.
Copyright ©2007 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like