WASHINGTON WRAP-UP
October 1, 2006
|
Although Utah Medical Products won a landmark FDA GMP/ QSR case a year ago, the company still has two strikes against it. First, FDA posted on its Web site documents that had been discredited by the federal judge in the case. Second, eventually, people get tired of hearing the same information and they tune out.
The company faced HHS again in July, when Utah Medical chairman and CEO Kevin L. Cornwell asked the department to reconsider its denial last February of the company's claim for administrative relief. The company had sought relief from “tortious abuse” by the agency employees who participated in the failed FDA case.
Utah Medical's Request The company's request to HHS for reconsideration outlines a number of remedies, including the following actions: • A posting of an FDA press release acknowledging that Utah Medical's quality system is and has been in compliance with the QSR (per the federal court's judgment). • A barring notice for the FDA inspectors and reviewers involved in earlier Utah Medical inspections. • The “purging of FDA's administrative file of all FDA-483s, EIRs, CDRH memos, interoffice e-mail, other fraudulent memoranda related to the 2001 through 2004 inspections and recommendations for permanent injunction.” • A commitment from the government to conduct and document the results of a formal investigation into FDA actions against the firm and to “retrain, reprimand, reassign, or dishonorably discharge FDA personnel who violated government rules and regulations.” |
Even as Cornwell did so, FDA posted the flawed documents to its Web site. They were the very same FDA-483s and EIRs that had formed the basis of the lawsuit. In an August 16 letter to FDA associate commissioner for regulatory affairs Margaret Glavin, Cornwell asked the agency to “immediately” withdraw the offending documents.
“The fact-finders and inspectors, who theoretically (although we know otherwise from documents obtained during discovery) authored the FDA-483s and EIRs, did not testify at trial for the reasons that the government should explain,” Cornwell wrote. “However, as I have previously expressed, these documents contain much in the text that is neither credible nor supportable.”
Cornwell told Glavin that Utah Medical had followed proper procedure before FDA sued it. The company showed that some of the documents contained misrepresentations and false statements.
“Although many other documents and deposition testimony contradict the content of the posted documents,” Cornwell wrote, “I believe that third-party visitors to your Web site will read them and attribute undeserved credibility to them. FDA posted these documents without properly disclosing that the issues identified in them were in fact adjudicated. As you know, the federal court's decision went completely against the FDA allegations, which were solely based on these documents. Now, to publish the documents without at least also publishing the court's decision, Utah Medical's responses, and other counterbalancing documentation seems to us to be an improper use of your authority to publicize, and I believe represents a continuing demonstration of FDA retaliation and intent to abuse Utah Medical.”
Utah Senator Orrin G. Hatch, who has stayed out of the long-running case until now, told the Salt Lake Tribune he had “contacted FDA officials at a very high level. I've asked them to review this issue and I'm confident that they will.” Cornwell also had earlier contacts with high-level FDA officials, including then-acting commissioner Lester Crawford on two occasions and Glavin predecessor Dennis Baker. Cornwell said he was promised reviews of his complaints that subsequent discovery documents and depositions revealed never occurred.
FDA has declined to comment on the matter. It cited the pending Utah Medical request for reconsideration as the reason for its silence.
Device Quality Pros Need More Support
The Ideal Organization Singer says she believes an ideal organization should have the following structure: • Firms that have many divisions and a corporate headquarters should give corporate staff oversight of division regulatory compliance activities. • Top officials for quality, regulatory, and compliance should be at the vice president level and equal in seniority to other officials in staff positions. • Top quality and compliance officials should report to the president and periodically address the board. • Firms should measure the cost of poor quality and invest sufficient resources in preventive action. • Firms should embrace risk management and use its concepts throughout their quality, regulatory, and decision-making processes. |
A Compliance-Alliance survey of 258 medical device firm professionals found that many professionals have the resources and authority to do their jobs. However, some do not, according to Compliance-Alliance president Nancy Singer. Singer said her firm conducted the survey in hopes that people would “use the results to convince their management to provide them with the necessary support to construct quality organizations that produce safe and effective healthcare products.”
The survey found that about half the firms organize their quality, regulatory, and compliance functions locally by manufacturing site, while the other half had corporate oversight. There was significant variation among firms on the question of which function has specific responsibilities.
Some 41% of quality, regulatory, and compliance groups report to a single point of control, and 28% have independent reporting structures. There was a wide variation in titles for the officials in those functions, with the most common title being vice president or senior vice president.
Experts who commented on the study results for Singer noted the importance of the top quality, regulatory, and compliance person being on the same level with the top finance, marketing, and R&D executives.
Most respondents said their quality, regulatory, and compliance officials report directly to the president, and 59% have the senior officials report regularly to the board of directors.
Most respondents (91%) reported their firms allow their top quality and regulatory personnel to stop production or order a recall. A majority of firms (67%) said that the regulatory function was responsible for product submissions, compliance to QSR, and ISO 13485. However, 37% said the regulatory function was responsible only for product registrations and licenses. The analysts said regulatory and quality are inseparable functions that need to be tightly coordinated to be effective.
When asked about commitment to quality and regulatory compliance, 27% of respondents said their firms strive to be the best in the industry. Sixty-two percent said their firms wanted to ensure product safety and efficacy and essential quality system compliance.
Finally, 7% reported wanting to minimally meet requirements. One analyst expressed disappointment that any company in the medical device business would only strive to meet minimum requirements. And another suggested an ideal company should want to be best in the business and an attractive place to work.
|
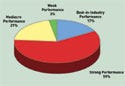
Figure 1. (click to enlarge) Survey respondents were asked to characterize the performance of their company's quality, regulatory, and compliance function. |
Asked to characterize performance of their quality, regulatory, and compliance function, 17% said they were among the best in the industry, and 59% said they had strong performance with no major nonconformities. Meanwhile, 21% reported mediocre performance with some major nonconformities in some areas, and 3% reported weak performance with major nonconformities (see Figure 1).
One analyst said the “brutal facts are that only a small percentage is truly among the best. Most are in the average-to-competent range, and a few are not effective. The problem is that many of the average-to-competent companies fail to recognize and accept their true status. Therefore, they fail to make the investments in people, infrastructure, and systems required to reach and maintain state of the art, let alone continuous improvements.”
Some 53% of the firms said they are not measuring the cost of poor quality (the cost of internal and external failures, appraisal, and preventive action). A majority of the firms said they are using risk-management principles throughout their quality system.
Violations Alleged at Teleflex
FDA says a two-month inspection at Teleflex Inc. (Limerick, PA) this past spring found CGMP problems in the firm's manufacture and distribution of Kirschner wires, Steinmann pins, and Hem-o-lok endoscopic ligating clip appliers.
A warning letter from the Atlanta District Office included various violations relating to acceptance criteria, corrective and preventive action procedures, and product validation. Also included were violations relating to conformance evaluation and statistical rationale for sampling methods.
The warning letter also said the firm could not provide validation data as to the adequacy of an ultrasonic aqueous degreasing and cleaning operation used on Kirschner wires and Steinmann pins. The investigator collected a variety of Kirschner wires and Steinmann pins during the inspection and had them analyzed. Some 49 of 59 pieces did not conform to release specifications. In addition, 67 of 156 pieces visually inspected contained defects. The Hem-o-lok clips were found to be misbranded because the company failed or refused to provide required information on corrections and removals.
FDA's letter acknowledged receipt of a response, but said it adequately addressed only some observations, while for several others it was inadequate. According to the warning letter, the agency's concerns with the initial response include the inadequacy of the investigation performed into Teleflex's nonconforming products. Also troublesome is the determination that there is no risk associated with the use of these devices and the lack of detail involving the expanded validation studies for the Hem-o-lok clip molding process. Finally, the agency cited its concern with the rationale for not reporting complaints under the Medical Device Reporting requirements.
Teleflex was told to take immediate action to correct the violations and submit a written report within 15 days on steps taken. The report is to include an explanation of how the firm plans to prevent these or similar violations from recurring.
Single-Tooth Anesthesia Device Cleared by FDA
FDA has given 510(k) clearance to Milestone Scientific's single-tooth anesthesia device. The computer-controlled local-anesthesia-delivery device provides audible and visual feedback to the dentist by measuring pressure at the tip of the needle. Company officials said the device is designed to improve efficiency and the economics of the dental office by anesthetizing only the tooth requiring repair.
The officials said that dentists often find single-tooth periodontal ligament injections difficult to administer and potentially painful for the patient. Therefore, dentists often use a mandibular block that anesthetizes one-quarter of the face around the tooth being worked on. But, they said, the company's CompuFlo pressure-measuring technology in the device enables dentists to use the feedback to administer injections accurately into the periodontal ligament space, effectively anesthetizing a single tooth.
FDA Challenges JAMA Article on Defibrillators
|
FDA has taken issue with an article stating that AED makers cannot track distributed units. |
FDA says an August 9 Journal of the American Medical Association (JAMA) article on automatic external defibrillator (AED) recalls was inaccurate. According to the agency, the article incorrectly stated that AED makers are unable to track distributed units and that therefore it is impossible to know how many devices were fixed or taken out of service. However, FDA's regulations require manufacturers to track AEDs and identify the location of a device in the event of a recall, an FDA notice says. “Our records show that these devices are being tracked with a high level of accuracy. In fact, more than 95% of the AEDs affected by Class I recalls in 2005 were returned to the manufacturers or taken out of service. Fewer than 3% were lost or stolen.”
FDA also takes issue with the JAMA authors' assertion that there has been an increase in the number of AEDs affected by product advisories during the study period. “This is true; however, FDA believes that improvements in the devices' ability to self-diagnose hardware and software problems may contribute to this trend,” the notice says. “This capability may result in users reporting problems before a device is ever used on a patient. Also, while more than 21% of AEDs were affected by an advisory, it does not necessarily mean that they malfunctioned. A device advisory is issued when a medical device has the potential to exhibit a certain failure mode, not only when a device has, in fact, failed.”
The JAMA article examines product advisories related to AEDs and their accessories. It finds that such advisories occur frequently and affect many devices. “Actual AED malfunctions do occur occasionally, although the number of observed malfunctions is small compared with the number of lives saved,” it says. During the study years 1996–2005, there were 52 product advisories affecting 385,922 AEDs and AED accessories, it says.
The study notes that advisories from FDA indicate the potential for a device to malfunction, and not an actual malfunction. “As such, they are a surrogate marker of device reliability. In general, FDA issues advisories when a product may not function as intended and the potential malfunction could result in patient harm. Importantly, some advisories are issued even when the risk of device failure is less than 1%. Timely communication of advisory information to device end-users is of critical importance, as some units may require repair or replacement.
“As more and more AEDs are deployed, the number of devices affected by product advisories can also be expected to increase,” the study concludes. “Efforts should be directed at developing a reliable system to locate and repair potentially defective devices in a timely fashion.”
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like