As the United States continues to debate the recently passed healthcare reform bill, China has put its own reform plan into action. The reform could represent significant opportunities for medical device manufacturers that are ready to find a niche in the land of the sleeping dragon.
May 6, 2010
In April 2009, the Chinese government announced the guidelines for healthcare reform, with the core principle of providing universal healthcare services to the country’s 1.3 billion population. From 2009 to 2011, China will invest 850 billion RMB ($124 billion USD) in healthcare. These developments are discussed in a report, “Analysis of the Chinese Healthcare Industry: A Guide for Medical Device Manufacturers,” created by InMedica (Wellingborough, Northamptonshire, UK).
|
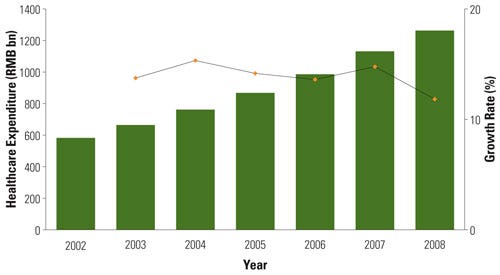
Healthcare expenditures in China have steadily increased. Source: Analysis of the Chinese Healthcare Industry, InMedica |
According to the report, grassroots-level hospitals and clinics are being given priority for development. The construction of centers as well as developing regulations are an opportunity for medical device OEMs, says Owen Tang, market analyst for InMedica.
Suppliers of basic equipment, such as general-purpose ultrasound machines, analog x-ray equipment, and patient monitors, all of which are funded by the reform, stand to gain significantly. “The reform offers opportunities for medical device companies with county-level hospitals, but those hospitals need a different type of equipment than the big cities do.
Companies that will see success are those that recognize needs, especially the price requirements,” Simon Harris, InMedica’s senior research director, medical group explains. “Companies that get it right stand to do pretty well.”
Multinational companies should prepare to work harder than Chinese companies to build their reputations. “China has additional regulations or requirements that apply to multinational companies, but do not apply to local suppliers,” Tang says.
For multinational firms, being part of the system is challenging, but not impossible. Companies should have some local presence, says Tang. “GE and Siemens, for example, have joint ventures or fully owned companies in China.”
Tang advises firms to be active in promoting their corporate citizenship in the county. “For example, they could train doctors in rural areas in using medical devices or provide donations (e.g., free breast cancer screening),” he says. “In this way, a company can get a reputation for social responsibility.”
For example, Tang says the Chinese government is looking into supplying rural facilities with CT devices and is running feasibility trials. “GE Healthcare has presented two new CT types that are designed for China users, with limited functions and a lower price. These devices are being provided specifically to serve rural hospitals.”
Tang also recommends building strong sales channels, especially in the county areas. “Setting up a good customer relationship with each hospital is vital, but it’s very difficult in rural areas. A distributor must be able to have a strong relationship with the decision makers in hospitals.”
China’s central government will fund the construction of 2000 county-level hospitals and 29,000 township hospitals, as well as the upgrading of 5000 township hospitals. Furthermore, about 3700 community health centers and 11,000 community health stations will be established or upgraded by 2011.
But 2011 is not the endgame. “Healthcare will continue to grow well beyond 2011,” Tang says. The reform represents a shift in Chinese culture that will continue to provide opportunities for medical device OEMs.
Attitudes are changing in China, explains Harris. “People are much more aware of health issues and are requesting better levels of care. They expect hospitals to have equipment and services. The demand for devices should be strong for quite a while.”
About the Author(s)
You May Also Like