Originally Published MDDI August 2006 CLEANING METHODSTraditionally, three techniques have been available for testing irregularly shaped devices to ensure that they have been cleaned adequately. An automated technique is also available. Here are the advantages and disadvantages of each.
August 1, 2006
CLEANING METHODS
|
Ensuring that components (parts) are free from contamination is becoming increasingly important in the medical device industry. Problems caused by inadequate or improper cleaning can harm patients and result in litigation, and, in some cases, business failure. Therefore, it is important to test parts once the cleaning process is complete to ensure that they have been cleaned adequately. That is, after being cleaned, parts need to be checked to see whether particles remain. Particle scanning devices can check the cleanliness of flat surfaces; however, such tools are not sufficient for medical devices that are complex in shape.
When testing the cleanliness of an irregularly shaped part (and therefore testing the effectiveness of the cleaning method), all methods follow two steps. The first is to extract the particles from the device into a liquid; the second is to test how many particles the liquid now contains. This article reviews the most common techniques used to test irregularly shaped devices and discusses the advantages and disadvantages of each.
Methods of Particle Extraction
The first step in testing the cleanliness of irregularly shaped parts is particle extraction. There are three traditional methods for this process: high-pressure spraying, sloshing or swirling, and ultrasonic extraction.
High-Pressure Spraying. In many cases, particle removal is accomplished by a simple spray wash. After the part has been cleaned, solvent or aqueous solution is sprayed from a high-pressure nozzle onto the surfaces of a part. The effluent is captured in a clean container as it runs off the part. This method is traditionally used when the contamination of interest is of relatively large size (>50 µm), when the size of the part is exceptionally large, or when the cleanliness requirement is minimal. Typically, the test is performed in an uncontrolled area with minimal filtration of the air.
For this method to be effective, it is imperative to know that both the cleaning solution and the collection vessel are clean. Even if using an exceptionally clean fluid such as ultrapure water, there is a risk of receiving a contaminated batch from the supplier, or a risk of a failure in the delivery mechanics contaminating the fluid. The particulate cleanliness of test fluid must be verified frequently enough to ensure its adequacy for the test. Ideally, the fluid should be verified before every test. As confidence builds in the system, the frequency could be reduced gradually. The point of this test is to ensure that the fluid-delivery system is not creating false high-particulate readings.
The second concern is the cleanliness of the effluent collection vessel. The collection vessel must be cleaned and checked before each test. Because the particle size of concern for this application is typically large, it is relatively easy to clean the test apparatus to produce the low background values necessary for a successful test. However, if vessel cleanliness were not verified before testing the part, a breakdown in the pretest cleaning process would go unnoticed. The particles in the dirty test vessel would increase the counts recorded during the actual part test. This could cause a clean part to be rejected when, in fact, it should be accepted.
Technique is another element that can vary in the high-pressure spray method. For example, suppose that one testing operator's interpretation of the job is to try to get as many particles off the part as possible. This individual painstakingly covers every millimeter of the part with slow, deliberate strokes. Another operator believes that the number of parts tested during the day is the most important indicator of job performance. This individual attempts to cover every millimeter with rapid strokes, believing that as long as the entire part is wet, a representative particles sample will be collected. Both of these operators have valid beliefs.
The first operator removes more particles from each part. The second operator processes more parts. As long as each operator maintains consistent technique, both generate data that are representative of the cleanliness of the parts. In other words, when parts are dirtier than normal, both show data with higher particle counts. However, it is difficult to compare the data produced by the two operators.
Usually, the data will be randomly mixed together, making it unclear that the differences can be attributed to operator technique. Combined with statistical variation in the cleanliness of the parts themselves, this creates the appearance that the cleanliness of the parts varies widely, even though both sets of data may show that the parts' cleanliness is within the required specifications. The fact that most of the parts are acceptable means that the upper control limit will be set quite high. This high limit may allow parts that are outside of acceptable levels to be passed on to manufacturing and packaging as clean parts.
Sloshing or Swirling. A sloshing or swirling method is typically used for smaller parts or to test containers for cleanliness. To test small parts, a clean container is filled with a carefully measured volume of clean solvent or aqueous cleaning fluid. The parts are added, and the operator then swirls the container of fluid for a designated interval of time. The number of particles added to the testing fluid determines the cleanliness of the part.
The sloshing or swirling extraction method suffers from the same shortcomings as the high-pressure spray method. All of the test materials must be verified as clean before each test. Because this method is often used for smaller particles, the extraction technique must be rigorous and followed carefully if reasonable backgrounds are to be achieved (<1% of expected contamination by part).
Operator technique influences the results. An operator with a more vigorous swirling or sloshing technique produces greater numbers of particles. In one experiment, the slosh test passed just as many uncleaned products as parts that had been through three cleaning steps. Although operator variance was the cause, an alternative conclusion could have been that the cleaning process was entirely ineffective.
Some users of the sloshing method have been known to standardize the method by using only one operator to perform the test. Both situations produce significant questions regarding the value of this method. If, when multiple operators are used, the test method cannot distinguish between a part that has been cleaned and one that has not been cleaned, it is unlikely that useful decisions can be made about the cleanliness of the parts. If only one operator can perform the test, problems can arise when that operator is not available. These problems must be addressed if this method is to become the primary qualification for the company's products.
Ultrasonic Extraction. Ultrasonics can be used for almost all materials, part sizes, and cleanliness levels. The procedure involves immersing the part in a bath containing a solvent or aqueous cleaning fluid. The bath has transducers bonded to the surface of the container that produce ultrasonic energy in the cleaning fluid.
This ultrasonic energy exerts significant cleaning power on the surface of the part. It is effective at removing even strongly bonded particles. Because the extraction force is constant, reliable and repeatable particle removal can be achieved. As with the previous two methods, the cleanliness of the test apparatus is critical. A method must be in place for testing the cleanliness of the test container complete with its test fluid. It is important to take this background measurement after exposing the test container and fluid to the ultrasonic energy.
Combining the more-efficient cleaning of ultrasonic energy with careful flushing of the container can achieve significantly lower background particle levels for the parts test. By achieving a lower background, the data track the parts' contamination level more accurately. This is critical to the performance of any of these methods. Inadequate background preparation produces results that are difficult to analyze.
A somewhat less obvious fault in this test (and in the swirl or slosh test) is determining the time for extraction. Most parts cleanliness tests use extraction intervals of <2 minutes; these tests require the operator to manually time the process. However, any interval longer than 30 seconds seems to be beyond the attention span of a typical operator, and a conversation between coworkers or other distraction can intervene, occasionally producing longer-than-expected extraction times. This potential distraction adds variability to the data, increasing the standard deviation. Automation of any kind can improve the results in this part of the test. At the very least, recurrent training is necessary to ensure that the operators maintain good technique and follow procedures closely to reduce data errors.
|
Table I. (click to enlarge) Summary of traditional methods of particle extraction. |
Summary of Extraction Methods. Table I summarizes the common extraction methods. Of the three methods described, only the ultrasonic method is highly repeatable, accurate, and precise. Human error is the greatest cause of error in all methods; the more hands-on time in the procedure, the more room for error.
Methods of Sample Analysis
After the particles have been extracted from the test part, they must be counted and sized, and then the results must be recorded for evaluation. To do this, the liquid that contains the extracted particles is analyzed. It is this analysis that provides information to manufacturers regarding whether the cleaning method was effective. Several methods can be used for counting the particles.
Gravimetric Analysis. Gravimetric analysis requires the use of a previously weighed membrane filter. The test fluid is pulled through the filter, then the filter is dried and reweighed. The additional mass of the filter is presumed to be from the particles extracted from the part. It is assumed that the particles are of the same density and size distribution as the original tests that were used to set acceptable mass levels.
Changes in particle density or size distribution produce data that could be extremely misleading. For instance, if stainless-steel particles are present in one test and nylon particles are present in another test, the gravimetric results will be quite different. Is the contaminated nylon part cleaner than the contaminated stainless-steel part? Is the contamination of nylon less important than the contamination of stainless steel? The answer to both questions is probably no. Additionally, with each sample transfer, care must be taken not to contaminate the test fluid or the membrane filter. Tests must be performed on the filters periodically to ensure that the drying process does not contaminate the test membrane. One careless action can ruin the entire test.
Optical Inspection. Optical inspection requires the use of a clean membrane filter. The test fluid is pulled through the filter. The filter is then placed on a microscope stage and inspected for particles larger than a specified dimension. Because of the magnification provided by the microscope, it is typical that only a small region of the filter is inspected.
In situations where parts are truly clean, there may only be a few damaging particles, which may be anywhere on the filter. How can one be assured that the location of these particles is included in the inspection region? Problems also occur in this method when particles are not uniform in shape. Particles can be measured over the longest dimension, average size, length of x-axis, etc. Different inspectors may use different methods or simply see the size differently, resulting in data variability. With each sample transfer, care must be taken not to contaminate the test fluid or the membrane filter.
Optical Particle Counter. An optical particle counter can also be used to sample the test fluid. Advantages of this method include rapid sampling capability, excellent sample-to-sample repeatability results in confidence in each test, accurate sizing information, and elimination of operator subjectivity and errors.
However, optical particle counters are not an absolute measure of particle size. They measure the equivalent optical size of the particle, reporting a value equivalent to a calibration particle. If the particle is similar to a fiber, i.e., long and thin, then orientation in the sample cell can produce different results. These limitations result in relatively little to no variation in the test results when compared with the possible variations produced during the extraction process.
|
Table II. (click to enlarge) Summary of methods of sample analysis. |
Summary of Traditional Analysis Methods. Of the three analysis methods, only the optical particle counter method eliminates human error and provides accurate, repeatable results (see Table II).
Testing Costs
The common themes in each of the above discussions of methods are time, labor, and equipment costs. Operators are involved in each step of the test. They must clean the sample test chambers, perform background checks, extract particles, and measure the contribution of the test parts.
As cleanliness requirements increase, measurements at smaller particle sizes are necessary, which increases the time needed to clean the apparatus. Because of the labor and time requirements, testing is typically limited to laboratory checks. The cleaned parts are removed from the cleaning system and sent to the lab for testing. The results of these tests may not be available for a day to a week later, depending on workload in the lab. For this reason, many industries only use lab checks when experiencing a major problem in yield.
Some manufacturers opt to skip such testing completely in favor of shotgun servicing of the cleaning equipment. The rationale is that by the time the results point to the actual problem, enough productivity would be lost to merit the expensive equipment maintenance.
The limitations of testing methods can produce extremely expensive repercussions when parts must be discarded or recalled to prevent reliability problems when customers use the product. Also, at best, these methods offer a very small sample of the overall control in the cleaning process. But the alternative requires a very large, full-time laboratory dedicated to parts testing.
Automated Technology
|
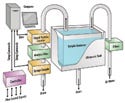
Figure 1. (click to enlarge) Automated technology incorporates ultrasonic extraction and particle counter technology. |
Automated technology incorporates ultrasonic extraction and particle counter technology, making it possible to move testing out of the laboratory. A typical system is shown in Figure 1. Automation reduces or eliminates the occurrence of human errors inherent in manual methods, and it helps maintain high throughput. Additionally, many advantages are realized by performing the testing right in the manufacturing area, when the parts exit the cleaning system.
Foremost, with automated technology, contaminated parts can be prevented from reaching the final stages of manufacturing. This reduces or eliminates product recall and rework caused by contamination failures. Additionally, increased information is available about the performance of the cleaning system.
Filter changes, bath changes, and major maintenance can be performed only when necessary, all of which increase the cleaning tool's uptime and utilization rates. Changes to the cleaning chemistry or routine can be immediately verified to determine whether the change offers a benefit or causes a detriment to the overall cleaning process. Permanently implementing changes that benefit the cleaning process leads to better results in manufacturing.
Conclusion
In the medical device industry, it is critical that components are free from contamination. It is essential to test parts to ensure that they have been cleaned adequately and that no particles remain once the cleaning is complete. Particle scanning devices can check the cleanliness of flat surfaces; however, these devices are not sufficient for medical devices that are complex in shape.
Manufacturers should understand the advantages and disadvantages of the most commonly used methods of extracting and analyzing particles to verify cleanliness. In particular, manufacturers need to ensure operator consistency and validate the apparatus cleanliness prior to testing parts. With these methods, both extraction and measurement require extensive manual labor and present major challenges.
Using an automated combination of methods provides cost savings brought about by real-time testing and eliminates much of the human error present in manual methods.
Dwight Beal is the service manager for Particle Measuring Systems (Boulder, CO). He can be reached at [email protected].
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like