In the first half of this century, research into materials synthesized from glycolic acid and other α-hydroxy acids was abandoned for further development because the resulting polymers were too unstable for long-term industrial uses. However, this very instability—leading to biodegradation—has proven to be immensely important in medical applications over the last three decades. Polymers prepared from glycolic acid and lactic acid have found a multitude of uses in the medical industry, beginning with the biodegradable sutures first approved in the 1960s.
Since that time, diverse products based on lactic and glycolic acid—and on other materials, including poly(dioxanone), poly(trimethylene carbonate) copolymers, and poly (ε-caprolactone) homopolymers and copolymers—have been accepted for use as medical devices. In addition to these approved devices, a great deal of research continues on polyanhydrides, polyorthoesters, polyphosphazenes, and other biodegradable polymers.
 A biodegradable intravascular stent prototype is molded from a blend of polylactide and trimethylene carbonate. Photo: Cordis Corp. Prototype Molded by Tesco Associates, Inc.
A biodegradable intravascular stent prototype is molded from a blend of polylactide and trimethylene carbonate. Photo: Cordis Corp. Prototype Molded by Tesco Associates, Inc.
Why would a medical practitioner want a material to degrade? There may be a variety of reasons, but the most basic begins with the physician's simple desire to have a device that can be used as an implant and will not require a second surgical intervention for removal. Besides eliminating the need for a second surgery, the biodegradation may offer other advantages. For example, a fractured bone that has been fixated with a rigid, nonbiodegradable stainless implant has a tendency for refracture upon removal of the implant. Because the stress is borne by the rigid stainless steel, the bone has not been able to carry sufficient load during the healing process. However, an implant prepared from biodegradable polymer can be engineered to degrade at a rate that will slowly transfer load to the healing bone. Another exciting use for which biodegradable polymers offer tremendous potential is as the basis for drug delivery, either as a drug delivery system alone or in conjunction to functioning as a medical device.
Polymer scientists, working closely with those in the device and medical fields, have made tremendous advances over the last 30 years. This article will focus on a number of these developments. We will also review the chemistry of the polymers, including synthesis and degradation, describe how properties can be controlled by proper synthetic controls such as copolymer composition, highlight special requirements for processing and handling, and discuss some of the commercial devices based on these materials.
POLYMER CHEMISTRY
Biodegradable polymers can be either natural or synthetic. In general, synthetic polymers offer greater advantages than natural materials in that they can be tailored to give a wider range of properties and more predictable lot-to-lot uniformity than can materials from natural sources. Synthetic polymers also represent a more reliable source of raw materials, one free from concerns of immunogenicity.
Polymer | Melting Point (°C) | Glass-Transition Temp (°C) | Modulus (Gpa)a | Degradation Time (months)b |
|---|---|---|---|---|
PGA | 225—230 | 35—40 | 7.0 | 6 to 12 |
LPLA | 173—178 | 60—65 | 2.7 | >24 |
DLPLA | Amorphous | 55—60 | 1.9 | 12 to 16 |
PCL | 58—63 | (—65)— (—60) | 0.4 | >24 |
PDO | N/A | (—10)— 0 | 1.5 | 6 to 12 |
PGA-TMC | N/A | N/A | 2.4 | 6 to 12 |
85/15 DLPLG | Amorphous | 50—55 | 2.0 | 5 to 6 |
75/25 DLPLG | Amorphous | 50—55 | 2.0 | 4 to 5 |
65/35 DLPLG | Amorphous | 45—50 | 2.0 | 3 to 4 |
50/50 DLPLG | Amorphous | 45—50 | 2.0 | 1 to 2 |
a Tensile or flexural modulus. | ||||
b Time to complete mass loss. Rate also depends on part geometry. |
Table I. Properties of common biodegradable polymers.
The general criteria for selecting a polymer for use as a biomaterial is to match the mechanical properties and the time of degradation to the needs of the application (see Table I). The ideal polymer for a particular application would be configured so that it:
Has mechanical properties that match the application, remaining sufficiently strong until the surrounding tissue has healed.
Does not invoke an inflammatory or toxic response.
Is metabolized in the body after fulfilling its purpose, leaving no trace.
Is easily processable into the final product form.
Demonstrates acceptable shelf life.
Is easily sterilized.
The factors affecting the mechanical performance of biodegradable polymers are those that are well known to the polymer scientist, and include monomer selection, initiator selection, process conditions, and the presence of additives. These factors in turn influence the polymer's hydrophilicity, crystallinity, melt and glass-transition temperatures, molecular weight, molecular-weight distribution, end groups, sequence distribution (random versus blocky), and presence of residual monomer or additives. In addition, the polymer scientist working with biodegradable materials must evaluate each of these variables for its effect on biodegradation.1
Biodegradation has been accomplished by synthesizing polymers that have hydrolytically unstable linkages in the backbone. The most common chemical functional groups with this characteristic are esters, anhydrides, orthoesters, and amides. We will discuss the importance of the properties affecting biodegradation later in the article.
The following section presents an overview of the synthetic biodegradable polymers that are currently being used or investigated for use in wound closure (sutures, staples); orthopedic fixation devices (pins, rods, screws, tacks, ligaments); dental applications (guided tissue regeneration); cardiovascular applications (stents, grafts); and intestinal applications (anastomosis rings). Most of the commercially available biodegradable devices are polyesters composed of homopolymers or copolymers of glycolide and lactide. There are also devices made from copolymers of trimethylene carbonate and ε-caprolactone, and a suture product made from polydioxanone.
Polyglycolide (PGA). Polyglycolide is the simplest linear aliphatic polyester. PGA was used to develop the first totally synthetic absorbable suture, marketed as Dexon in the 1960s by Davis and Geck, Inc. (Danbury, CT). Glycolide monomer is synthesized from the dimerization of glycolic acid. Ring-opening polymerization yields high-molecular-weight materials, with approximately 1—3% residual monomer present (see Figure 1). PGA is highly crystalline (45—55%), with a high melting point (220—225°C) and a glass-transition temperature of 35—40°C. Because of its high degree of crystallization, it is not soluble in most organic solvents; the exceptions are highly fluorinated organics such as hexafluoroisopropanol. Fibers from PGA exhibit high strength and modulus and are too stiff to be used as sutures except in the form of braided material. Sutures of PGA lose about 50% of their strength after 2 weeks and 100% at 4 weeks, and are completely absorbed in 4—6 months. Glycolide has been copolymerized with other monomers to reduce the stiffness of the resulting fibers.
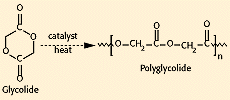 Figure 1. Synthesis of polyglycolide (PGA).
Figure 1. Synthesis of polyglycolide (PGA).
Polylactide (PLA). Lactide is the cyclic dimer of lactic acid that exists as two optical isomers, d and l. l-lactide is the naturally occurring isomer, and dl-lactide is the synthetic blend of d-lactide and l-lactide. The homopolymer of l-lactide (LPLA) is a semicrystalline polymer. These types of materials exhibit high tensile strength and low elongation, and consequently have a high modulus that makes them more suitable for load-bearing applications such as in orthopedic fixation and sutures. Poly(dl-lactide) (DLPLA) is an amorphous polymer exhibiting a random distribution of both isomeric forms of lactic acid, and accordingly is unable to arrange into an organized crystalline structure. This material has lower tensile strength, higher elongation, and a much more rapid degradation time, making it more attractive as a drug delivery system. Poly(l-lactide) is about 37% crystalline, with a melting point of 175—178°C and a glass-transition temperature of 60—65°C. The degradation time of LPLA is much slower than that of DLPLA, requiring more than 2 years to be completely absorbed. Copolymers of l-lactide and dl-lactide have been prepared to disrupt the crystallinity of l-lactide and accelerate the degradation process.
Poly(ε-caprolactone). The ring-opening polymerization of ε-caprolactone yields a semicrystalline polymer with a melting point of 59—64°C and a glass-transition temperature of —60°C (see Figure 2). The polymer has been regarded as tissue compatible and used as a biodegradable suture in Europe. Because the homopolymer has a degradation time on the order of 2 years, copolymers have been synthesized to accelerate the rate of bioabsorption. For example, copolymers of ε-caprolactone with dl-lactide have yielded materials with more-rapid degradation rates. A block copolymer of ε-caprolactone with glycolide, offering reduced stiffness compared with pure PGA, is being sold as a monofilament suture by Ethicon, Inc. (Somerville, NJ), under the trade name Monacryl.
 Figure 2. Synthesis of poly(ε-caprolactone).
Figure 2. Synthesis of poly(ε-caprolactone).
Poly(dioxanone) (a polyether-ester). The ring-opening polymerization of p-dioxanone (see Figure 3) resulted in the first clinically tested monofilament synthetic suture, known as PDS (marketed by Ethicon). This material has approximately 55% crystallinity, with a glass-transition temperature of —10 to 0°C. The polymer should be processed at the lowest possible temperature to prevent depolymerization back to monomer. Poly(dioxanone) has demonstrated no acute or toxic effects on implantation. The monofilament loses 50% of its initial breaking strength after 3 weeks and is absorbed within 6 months, providing an advantage over Dexon or other products for slow-healing wounds.
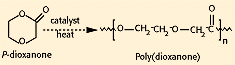 Figure 3. Synthesis of poly(dioxanone).ε-caprolactone).
Figure 3. Synthesis of poly(dioxanone).ε-caprolactone).
Poly(lactide-co-glycolide). Using the polyglycolide and poly(l-lactide) properties as a starting point, it is possible to copolymerize the two monomers to extend the range of homopolymer properties (see Figure 4). Copolymers of glycolide with both l-lactide and dl-lactide have been developed for both device and drug delivery applications. It is important to note that there is not a linear relationship between the copolymer composition and the mechanical and degradation properties of the materials. For example, a copolymer of 50% glycolide and 50% dl-lactide degrades faster than either homopolymer (see Figure 5). Copolymers of l-lactide with 25—70% glycolide are amorphous due to the disruption of the regularity of the polymer chain by the other monomer. A copolymer of 90% glycolide and 10% l-lactide was developed by Ethicon as an absorbable suture material under the trade name Vicryl. It absorbs within 3—4 months but has a slightly longer strength-retention time.
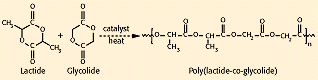 Figure 4. Synthesis of poly(lactide-co-glycolide).ε-caprolactone).
Figure 4. Synthesis of poly(lactide-co-glycolide).ε-caprolactone).
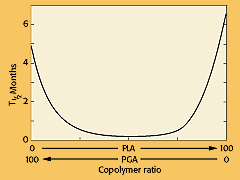 Figure 5. Half-life of PLA and PGA homopolymers and copolymers implanted in rat tissue. (Figure reproduced courtesy of Journal of Biomedical Materials Research, 11:711, 1977.)
Figure 5. Half-life of PLA and PGA homopolymers and copolymers implanted in rat tissue. (Figure reproduced courtesy of Journal of Biomedical Materials Research, 11:711, 1977.)
Copolymers of glycolide with trimethylene carbonate (TMC), called polyglyconate (see Figure 6), have been prepared as both sutures (Maxon, by Davis and Geck) and as tacks and screws (Acufex Microsurgical, Inc., Mansfield, MA). Typically, these are prepared as A-B-A block copolymers in a 2:1 glycolide:TMC ratio, with a glycolide-TMC center block (B) and pure glycolide end blocks (A). These materials have better flexibility than pure PGA and are absorbed in approximately 7 months. Glycolide has also been polymerized with TMC and p-dioxanone (Biosyn, by United States Surgical Corp., Norwalk, CT) to form a terpolymer suture that absorbs within 3—4 months and offers reduced stiffness compared with pure PGA fibers.
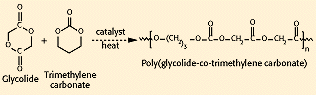 Figure 6. Synthesis of polyglyconate.
Figure 6. Synthesis of polyglyconate.
Other Polymers under Development. Currently, only devices made from homopolymers or copolymers of glycolide, lactide, caprolactone, p-dioxanone, and trimethylene carbonate have been cleared for marketing by FDA. A number of other polymers, however, are being investigated for use as materials for biodegradable devices.
In addition to their suitability for medical uses, biodegradable polymers make excellent candidates for packaging and other consumer applications. A number of companies are evaluating ways to make low-cost biodegradable polymers. One method is to bioengineer the synthesis of the polymers, using microorganisms to produce energy-storing polyesters. Two examples of these materials—polyhydroxybutyrate (PHB) and polyhydroxyvalerate (PHV)—are commercially available as copolymers under the trade name Biopol (Monsanto Co., St. Louis) and have been studied for use in medical devices (see Figure 7). The PHB homopolymer is crystalline and brittle, whereas the copolymers of PHB with PHV are less crystalline, more flexible, and easier to process. These polymers typically require the presence of enzymes for biodegradation but can degrade in a range of environments and are under consideration for several biomedical applications.
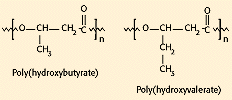 Figure 7. Molecular structure of two bioengineered polyesters that require specific enzymes for biodegradation.
Figure 7. Molecular structure of two bioengineered polyesters that require specific enzymes for biodegradation.
The use of synthetic poly(amino acids) as polymers for biomedical devices would seem a logical choice, given their wide occurrence in nature. In practice, however, pure insoluble poly(amino acids) have found little utility because of their high crystallinity, which makes them difficult to process and results in relatively slow degradation. The antigenicity of polymers with more than three amino acids in the chain also makes them inappropriate for use in vivo. To circumvent these problems, modified "pseudo" poly(amino acids) have been synthesized by using a tyrosine derivative. Tyrosine-derived polycarbonates, for example, are high-strength materials that may be useful as orthopedic implants. It is also possible to copolymerize poly(amino acids) to modify their properties. The group that has been researched most extensively is the polyesteramides.
A Note on Nomenclature
A polymer is generally named based on the monomer it is synthesized from. For example, ethylene is used to produce poly(ethylene). For both glycolic acid and lactic acid, an intermediate cyclic dimer is prepared and purified, prior to polymerization. These dimers are called glycolide and lactide, respectively. Although most references in the literature refer to polyglycolide or poly(lactide), you will also find references to poly(glycolic acid) and poly(lactic acid). Poly(lactide) exists in two stereo forms, signified by d or l for dexorotary or levorotary, or by dl for the racemic mix.
The search for new candidate polymers for drug delivery may offer potential for medical device applications as well. In drug delivery, the formulation scientist is concerned not only with shelf-life stability of the drug but also with stability after implantation, when the drug may reside in the implant for 1—6 months or more. For drugs that are hydrolytically unstable, a polymer that absorbs water may be contraindicated, and researchers have begun evaluating more hydrophobic polymers that degrade by surface erosion rather than by bulk hydrolytic degradation. Two classes of these polymers are the polyanhydrides and the polyorthoesters.
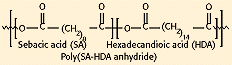 Figure 8. Molecular structure of poly(SA-HDA anhydride).
Figure 8. Molecular structure of poly(SA-HDA anhydride).
Polyanhydrides have been synthesized via the dehydration of diacid molecules by melt polycondensation (see Figure 8). Degradation times can be adjusted from days to years according to the degree of hydrophobicity of the monomer selected. The materials degrade primarily by surface erosion and possess excellent in vivo compatibility. So far, they have only been approved for sale as a drug delivery system. The Gliadel product, designed for delivery of the chemotherapeutic agent BCNU in the brain, received regulatory clearance from FDA in 1996 and is being produced by Guilford Pharmaceuticals, Inc. (Baltimore).
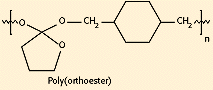 Figure 9. Molecular structure of poly(orthoester).
Figure 9. Molecular structure of poly(orthoester).
Polyorthoesters were first investigated in the 1970s by Alza Corp. (Palo Alto, CA) and SRI International (Menlo Park, CA) in a search for new synthetic biodegradable polymers for drug delivery applications (see Figure 9). These materials have gone through several generations of improvements in synthesis, and can now be polymerized at room temperature without forming condensation by-products. Polyorthoesters are hydrophobic, with hydrolytic linkages that are acid-sensitive but stable to base. They degrade by surface erosion, and degradation rates can be controlled by incorporation of acidic or basic excipients.
PACKAGING AND STERILIZATION
Because biodegradable polymers are hydrolytically unstable, the presence of moisture can degrade them in storage, during processing, and after device fabrication. In theory, the solution for hydrolysis instability is simple: eliminate the moisture and thus eliminate the degradation. However, because the materials are naturally hygroscopic, eliminating water and then keeping the polymer free of water are difficult to accomplish. The as-synthesized polymers have relatively low water contents, since any residual water in the monomer is used up in the polymerization reaction. The polymers are quickly packaged after manufacture—generally double-bagged under an inert atmosphere or vacuum. The bag material may be polymeric or foil, but it must be highly resistant to water permeability. To minimize the effects of any moisture present, the polymers are typically stored in a freezer. Packaged polymers should always be at room temperature when opened to minimize condensation, and should be handled as little as possible at ambient atmospheric conditions. As expected, there is a relationship among biodegradation rate, shelf stability, and polymer properties. For instance, the more hydrophilic glycolide polymers are much more sensitive to hydrolytic degradation than are polymers prepared from the more hydrophobic lactide.
Final packaging consists of placing the suture or device in an airtight, moistureproof container. A desiccant can be added to further reduce the effects of moisture. Sutures, for example, are wrapped around a specially dried paper holder that acts as a desiccant. In some cases, the finished device may be stored at subambient temperature as an added precaution against degradation.
Devices incorporating biodegradable polymers cannot be subjected to autoclaving, and must be sterilized by gamma or E-beam irradiation or by exposure to ethylene oxide (EtO) gas. There are certain disadvantages, however, to both irradiation and EtO sterilization. Irradiation, particularly at doses above 2 Mrd, can induce significant degradation of the polymer chain, resulting in reduced molecular weight as well as influencing final mechanical properties and degradation times. Polyglycolide, poly(lactide), and poly(dioxanone) are especially sensitive to ionizing radiation, and these materials are usually sterilized by EtO for device applications. Because the highly toxic EtO can present a safety hazard, great care must be taken to ensure that all the gas is removed from the device before final packaging. The temperature and humidity conditions should also be considered when submitting devices for sterilization. Temperatures must be kept below the glass-transition temperature of the polymer to prevent the part geometry from changing during sterilization. If necessary, parts can be kept at 0°C or lower during the irradiation process.
PROCESSING
All commercially available biodegradable polymers can be melt processed by conventional means such as injection molding, compression molding, and extrusion. As with packaging, special consideration needs to be given to the exclusion of moisture from the material before melt processing to prevent hydrolytic degradation. Special care must be taken to dry the polymers before processing and to rigorously exclude humidity during processing.
Because most biodegradable polymers have been synthesized by ring-opening polymerization, a thermodynamic equilibrium exists between the forward or polymerization reaction and the reverse reaction that results in monomer formation. Excessively high processing temperatures may result in monomer formation during the molding or extrusion process. The presence of excess monomer can act as a plasticizer, changing the material's mechanical properties, and can catalyze the hydrolysis of the device, thus altering degradation kinetics. Therefore, these materials should be processed at the lowest temperatures possible.
About the Author(s)
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)