August 1, 2009
News Trends
Decoding the Ethics Code Survey
|
A recent survey conducted by PricewaterhouseCoopers (PwC)concluded that the medical device industry has made significant efforts to enact ethical policies. According to the survey, 90% of respondents, representing 32 companies, have policies in place that address all provisions of the AdvaMed Code of Ethics.
The survey results, although small, cover typical demographics of medical device companies, explains Deepa Gupta, senior scientist for the Cardiac Rhythm Management group at Boston Scientific Inc. She says the sample represents industry appropriately. “[The survey] gives us an opportunity to see how they are doing in terms of compliance with regard to other medical device companies.”
Some highlights of the study show that respondents continue to expand ethics and compliance programs. For example, three-fourths of respondents indicated that the CEO shows support for the AdvaMed Code, or its corresponding company policies, by participating in company activities, such as making statements to employees at live meetings, writing statements in a letter to employees, and sending statements in an e-mail. For the most part, a board or board committee oversees compliance, with more than half (65%) having written policies and procedures, or an established practice for notifying the board of compliance-related matters.
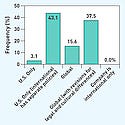
Medical device firms also strive to ensure that acquisitions are in compliance, either within 90 days of the acquisition (63%) or immediately (25%).
|
(click to enlarge) |
One anticipated challenge to ethics adoption is gaining acceptance from device OEMs' customers. However, the study indicates that such acceptance is not always a significant hurdle. Participants noted that 44% of customers reacted with at least partial acceptance, while 19% noted some resistance, and only 3% said they had met substantial resistance from customers.
That said, companies are still finding it a challenge to identify key performance indicators (that is, tracking and measuring the effectiveness of their compliance programs). In two other recent surveys conducted by PwC, a majority of companies indicated that they had few or no formal performance indicators.
Despite such challenges, the study results indicate significant adoption of ethics and compliance within medical device manufacturing organizations. Doug Mowen, advisory leader of the medical device industry practice at PwC, says OEMs have made a “clear commitment to ethical business practices through adoption of the Code, putting openness and transparency on their relationships with healthcare providers at the forefront. Ultimately, this benefits patients by removing any perceived bias, thus increasing the quality of care.”Copyright ©2009 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like