November 14, 2004
Originally Published MPMN November 2004
PROFILE
Service Provides Process Improvement for OEMs
Process problems can be solved quickly
Joyce Laird
|
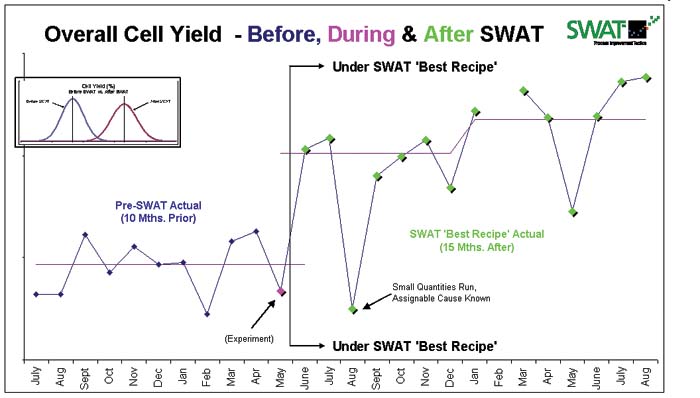
This graph shows output results for a process that was optimized for W.L. Gore & Associates Medical Products Div (click to enlarge). |
Processing problems are often a challenge for medical device manufacturers. How does one improve a process that is essentially frozen as is by product and process compliance restrictions?
W.L. Gore & Associate’s Medical Products Div. (Flagstaff, AZ; www.goremedical.com) faced this situation recently with one of its emerging implantable devices. The product had been an underperformer, in terms of production yield, from its inception. The company tried all the obvious remedies, but yield performance remained less than adequate.
This is when it called InSite Technologies Inc. (North East, MD; www.insitetechnologies.net) for help. InSite is a consulting firm that specializes in fast problem-process solutions. The company offers an approach to production floor experimentation and monitoring called SWAT process improvement tactics. An analysis using SWAT provides rapid gains in knowledge through the concurrent use of large-scale, statistically designed experiments and a process-monitoring regimen.
To solve Gore’s specific problem, InSite first assembled a cross-functional group of employee process experts made up of operators, engineers, supervisors, maintenance technicians, and scientists. Within three hours, InSite helped the team identify 187 blue-sky ideas to improve production yield.
Over the next day, the team reviewed each of these ideas for practicality and cost feasibility. Twenty-one of them were deemed immediately doable on goods available for shipment. All the ideas cost nothing to test and implement, and were within required product and process specifications.
The potential solutions involved slight changes in tensions, diameters, thicknesses, strengths, widths, targets, speeds, positions, and temperatures. Basically, any parameter that could vary while remaining within regulated operating conditions was tested. For example, the team asked might wire diameter matter? Or more accurately, might differences in wire diameter, allowed within the specification, impact yield? If so, would higher be better, and by how much?
As part of the SWAT process, a statistically based experiment was designed to accommodate these 21 ideas. This called for the running of 48 unique combinations or process recipes that would enable yield performance to be predicted for more than 2 million combinations of these variable conditions. This included learning about 210 two-factor interactions as well. A two-factor interaction occurs when the influence a process input has on a process output is dependent on the setting of another input.
Over the next few weeks, InSite oversaw and monitored hundreds of devices produced under the 48 experimental combinations. All of the units were production-worthy articles, but they were also performing double duty as “experimental units.” Yield during this time was higher on two subassemblies but slightly lower overall. Shipping volumes were comparable to those normally achieved.
Once combinations were complete, InSite conducted statistical analyses to determine the best recipe among the two million plus recipes studied. This was done for process yield as well as 19 other measures of product and process performance.
InSite’s newly uncovered Best Recipe was forecast to provide a double-digit yield increase. This was driven primarily by three readily implementable changes to Gore’s standard operating procedure. The changes involved included using only the high end of the allowable range for a particular raw material attribute, using a lower target value in a certain processing step, and using a slightly slower equipment speed setting.
“Large factor quantities and large sample sizes provide the keys to unlocking actionable information from processes such as this,” says Ron Fritz, PhD, founder of InSite Technologies Inc. “They provide the comprehensiveness and statistical power to discern cause from coincidence with such small differences in tested settings.”
InSite’s prescribed best recipe was immediately implemented and provided the forecasted double-digit increase in performance. The increase has been sustained for a year now, and demonstrates what access to intimate process knowledge can do for frozen-as-is processes so common in the medical device industry.
Copyright ©2004 Medical Product Manufacturing News
You May Also Like